Ethylene oxide (EO) is a versatile chemical building block. It's currently produced through ethylene epoxidation over supported silver catalysts at 200–260 °C and 1–3 MPa using O2 as an oxygen source. This process produces substantial CO2 which originates from the complete oxidation of ethylene and elevated temperatures and pressures powered by fossil fuels.
Halide-mediated electrocatalytic ethylene epoxidation reaction under mild conditions offers a low-carbon and sustainable route for EO production. However, the strongly acidic, corrosive, and oxidative reaction environments lead to severe dissolution and deactivation of catalytic materials, even for noble metal catalysts.
Recently, a research team led by Profs. BAO Xinhe, WANG Guoxiong, and GAO Dunfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have proposed an inverse single-atom modulation strategy to develop active and stable catalysts for chloride-mediated electrocatalytic ethylene epoxidation to EO.
This study was published in Angewandte Chemie International Edition on Mar. 21.
Boosting electrocatalytic ethylene epoxidation by single atom modulation (Image by WANG Hanyu)
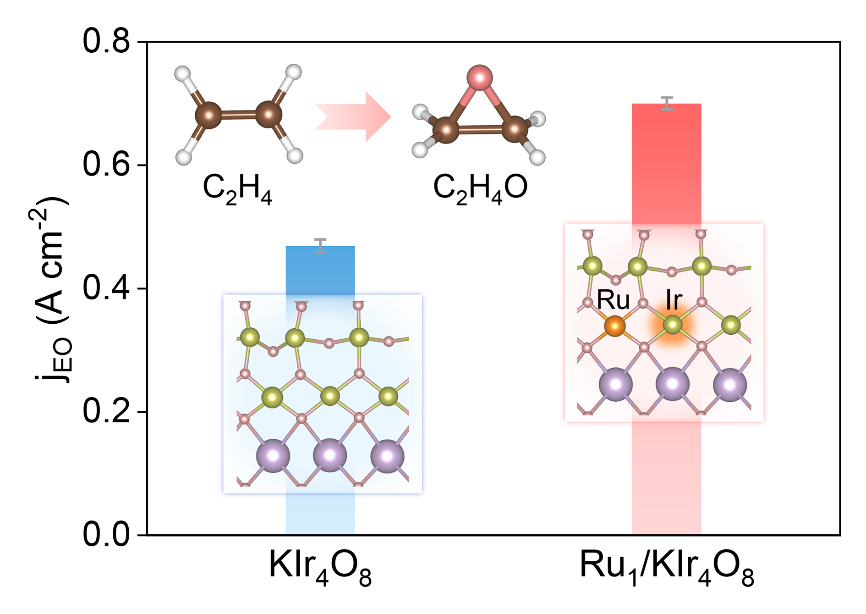
The researchers developed a single-atom Ru-doped KIr4O8 (KIrRuO) nanowire catalyst by substituting a portion of Ir atoms in the hollandite structure KIr4O8 with doped Ru atoms. The KIrRuO catalyst exhibited an EO partial current density of up to 0.7 A/cm2 and an EO yield as high as 92%.
The impressive ethylene epoxidation performance was ascribed to the modulation of electronic structures of adjacent Ir sites by single Ru atoms, which stabilized the *CH2CH2OH intermediate and facilitates the formation of active Cl2 species during the generation of 2-chloroethanol, the precursor of EO.
"Our work provides a single-atom modulation strategy for tuning the reactivity of adjacent metal sites in heterogeneous electrocatalysts," said Prof. GAO.
"This work also expands reaction routes for the electrocatalytic conversion of carbon-based resources," said Prof. WANG.
The above work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Liao Ning Revitalization Talents Program, and the Youth Innovation Fund of DICP.