Transition metal hydrides (TMHs) can be used as high-efficiency catalysts for hydrogen-involving reactions due to their finely modulable electronic states and reaction paths by interstitial hydrogen.
However, their severe thermodynamic instability brings challenges for preparation and utilization under ambient conditions.
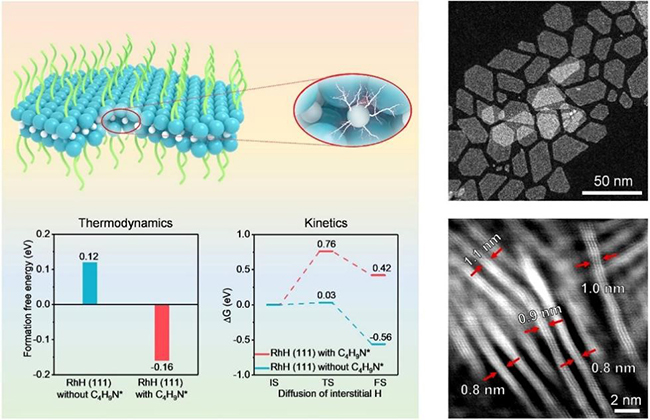
Structural properties of surface ligand-confined 2D rhodium hydride (Image by FAN Jinchang)
Recently, a research group led by Prof. DENG Dehui and Assoc. Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) constructed novel ambient-stable two-dimensional (2D) TMHs which are rhodium hydride (RhH) nanosheets.
This study was published in Matter on Sep. 28.
TMHs are usually thermodynamically unstable with positive formation free energies under standard conditions and thus can only be formed under immense H2 pressure (at least several GPa) or highly reductive conditions.
So far, palladium hydride is the only-reported ambient-stable TMH owing to its relatively strong interaction between Pd and H, while preparation and utilization of novel Pd-free ambient-stable TMHs under mild conditions is still unachieved.
In this study, the researchers used electron-attracting n-butylamino ligands to enhance the interaction between Rh and interstitial hydrogen, making the formation of 2D RhH thermodynamically permissible. It exhibited a superior hydrogen evolution reaction (HER) activity with a lower overpotential and 3.1 times and 1.4 times higher mass activity than 2D Rh catalyst and commercial Pt/C catalyst, respectively.
Moreover, the theoretical calculations revealed the significant effect of interstitial H atoms and surface ligands in boosting the HER activity of Rh, by moderately weakening hydrogen adsorption and accelerating H2 desorption via a ligand-mediated H-transfer mechanism
This work was supported by the National Natural Science Foundation of China, National Key R&D Program of China and the Young Cross Team Project of CAS.