Hydrogenation of carbon dioxide (CO2) to formate, an important chemical widely used in leather, textile, mining industry, etc., is an attractive atom-economic route for the utilization of this greenhouse gas.
Non-precious metal-based catalysts for the CO2 hydrogenation to formate suffered from either low activity or low stability. It's still a challenge to develop low-cost and high-performance catalysts for CO2 hydrogenation to formate.
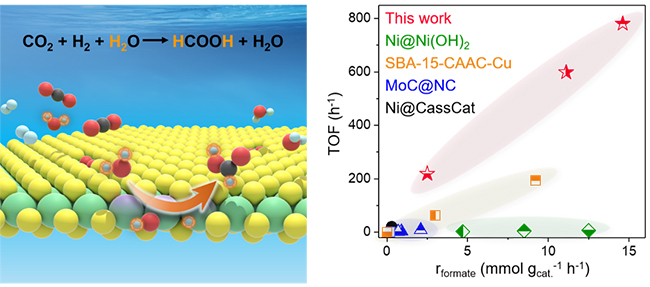
Recently, a research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed an edge-rich molybdenum disulfide (ER-MoS2) catalyst for CO2 hydrogenation to formate with superior activity and high stability.
The study was published in Angew. Chem. Int. Ed. on July 20 and was selected as hot paper and inside back cover.
ER-MoS2 as catalyst for the hydrogenation of CO2 to formate (Image by WANG Zifeng and YU Liang)
In this study, the ER-MoS2 with abundant edges delivered a high TOF of 780.7 h-1 with formate selectivity of over 99% at 200 °C, and exhibited a good stability.
Multiple experimental characterizations combined with theoretical calculations revealed that sulfur vacancies at MoS2 edges were the active sites and the selective production of formate was enabled via a new water-mediated hydrogenation mechanism, in which surface OH* and H* species from H2O dissociation on the edge-sulfur vacancies served as moderate hydrogenating agents with residual O* reduced by H2.
"This work opens new avenue for developing low-cost non-noble metal catalysts for the hydrogenation of CO2 to formate and the revealed water-mediated reaction mechanism also provides insights for designing MoS2-based catalysts for selective hydrogenation reactions," said Prof. DENG.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of CAS, the LiaoNing Revitalization Talents Program, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM).