Selective hydrogenation of carbon monoxide (CO) to higher alcohols (C2+OH) is a promising non-petroleum route for producing high-value chemicals, in which precise regulations of both C-O cleavage and C-C coupling are highly essential but remain challenges.
Recently, a research group led by Prof. DENG Dehui and Assoc. Prof. YU Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. WANG Ye from Xiamen University, realized highly selective CO hydrogenation to C2-4OH over a potassium-modified edge-rich molybdenum disulfide (ER-MoS2-K) catalyst.
This study was published in Nature Communications on Oct. 26.
Tunable CO hydrogenation selectivity over MoS2-based catalyst (Image by HU Jingting and WEI Zeyu)
The ER-MoS2-K catalyst, which was assembled in nano-array morphology with uniform linear channels, was prepared on the basis of a nano channel-confined growth mechanism.
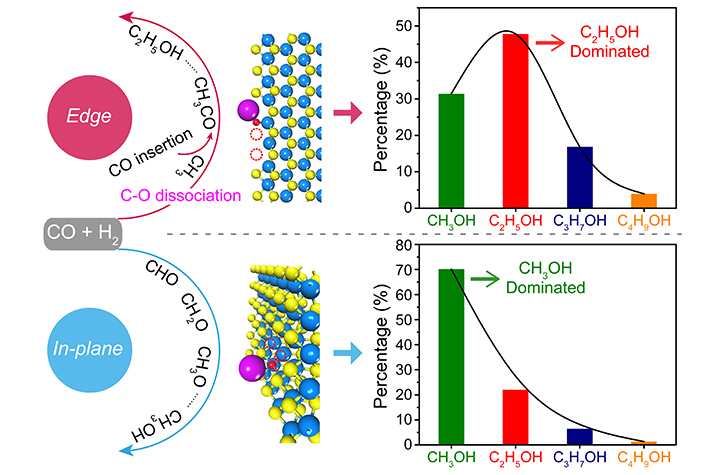
The researchers found that it could deliver a high CO conversion of 17% with a superior C2-4OH selectivity of 45.2% in hydrogenated products at 240 oC and 50 bar. Moreover, by reducing the lateral size of MoS2 to enrich the edges for boosting the carbon-chain growth, the researcher achieved the C2-4OH to methanol selectivity ratio overturn from 0.4 to 2.2, and the selectivity of C2-4OH could reach to over 99% in the C2+OH products.
In-situ characterizations combined with theoretical calculations revealed that the sulfur vacancies (SVs) at the edge of MoS2 boosted carbon-chain growth by facilitating simultaneously the C-O cleavage of CHxO* for generating CHx* intermediate, and the subsequent C-C coupling between CO* and CHx*, while the potassium promoter promoted the desorption of alcohols via electrostatic interaction with hydroxyls, thereby enabling controllable formation of C2-4OH.
"This work presents the high flexibility of edge SVs of MoS2 in tailoring both C-O cleavage and C-C coupling for carbon-chain growth in CO hydrogenation, thus providing a prototype for the rational design of the nanostructure and microenvironment of active sites for selective hydrogenation reactions," said Prof. DENG.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Strategic Priority Research Program of CAS, and the Fundamental Research Funds for the Central Universities.