The water‐gas shift (WGS) reaction (CO+H2O→CO2+H2) is critical in producing high‐purity hydrogen for ammonia and methanol synthesis as well as in fuel cell applications.
By the concept of single‐atom catalysts (SAC), scientists now can understand the chemical bond and electronic structure between metal and support in an atomic scale.
A research group led by Prof. WANG Xiaodong and Prof. ZHANG Tao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LI Jun from Tsinghua University, proposed a redox mechanism with synergetic dual metal active sites for the WGS reaction on an Ir1/FeOx SAC.
The results were published in Angew. Chem. Int. Ed.
The scientists reported a combined theoretical and experimental study on SAC of Ir1/FeOx for WGS reaction. They found that water easily dissociated to OH* on the Ir1 single atom and H* on the nearby O atom bonded with a Fe site. The adsorbed CO on Ir1 reacts with the adjacent O atom to produce CO2. Then, the formation of H2 became feasible due to migration of H from adsorbed OH* toward Ir1 and its subsequent reaction with another H*.
They proposed a new pathway of a redox mechanism via synergetic dual metal active sites between Ir1 and Fe species (DMAS) during the WGS reaction, which demonstrated the sequential production of CO2 and H2.
The elucidation of the catalytic mechanism involving this kind of dual metal active sites might provide insights for understanding the catalytic mechanism of metal catalysts with reducible supports, thus helping rational design of new and active dual-site single-atom and single-cluster catalysts.
This work was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of CAS. (Text by LIN Jian)
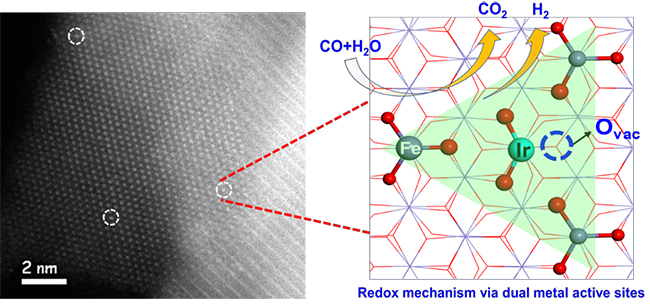
Synergetic dual metal active sites of Ir1/FeOx SAC for WGS reaction (Image by LIN Jian)