Recently, Dr. ZHOU Yan and Prof. SHEN Wenjie at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and their collaborators identified the atomic structure of the catalytically active copper-ceria interface and proposed a copper bilayer model. Their findings were published in Nature Catalysis.
Because of their high natural abundance, low cost, and, more importantly, their unique electronic characteristics, copper catalysts have been industrially applied and fundamentally studied for several chemical reactions that are intimately relevant to energy.
Copper nanoparticles, dispersed on ceria, constitute a highly efficient catalyst system for the low-temperature water-gas shift reaction, which generates hydrogen, and CO/CO2 hydrogenation that yields methanol. The two processes are crucial for utilizing carbon resources.
The copper-ceria interface has been presumed responsible for the catalytic performance. However, direct identification and a quantitative description of the active sites, which directly interact with the reactive molecules during catalysis, remain challenging.
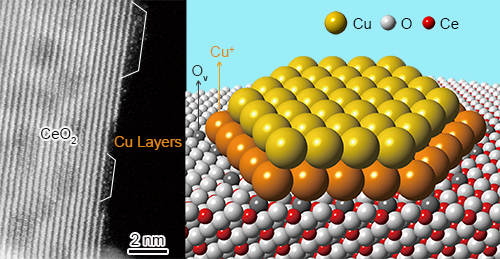
Atomic structure of the copper-ceria interface. (Image by ZHOU Yan)
"Our conclusions are based on the observation of tiny copper clusters by a combination of scanning transmission electron microscopy (STEM) and electron energy loss spectroscopy (EELS), probing the interfacial bonding environment by in situ infrared (IR) spectroscopy, and rationalization by density functional theory (DFT) calculations," said Prof. SHEN.
"In studying the atomic structure of the catalytically active copper-ceria interface, we found the copper cluster consisted of a bottom layer of mainly Cu+ atoms bonded on the oxygen vacancies of ceria, and a top layer of Cu0 atoms coordinated with the underlying Cu+ atoms," Prof. SHEN added.
Moreover, the scientists found that the low-temperature water-gas shift reaction occurred at the copper-ceria interfacial perimeter via a site cooperation mechanism, by which the Cu+ site chemically adsorbs CO while the neighboring oxygen vacancy site dissociatively activates water. (Text by ZHOU Yan)