Chemical reactions on solid catalyst surfaces are complicated with high-dimensional variables. A better way for catalyst design is the establishment of activity and selectivity trends over a collection of catalysts.
The traditional design scheme is usually based on pre-assumed reaction pathways, and it's difficult to solve microkinetic models as there are thousands of reaction pathways. Therefore, it is still highly risky to design a new catalytic reaction prior to experimental studies.
Recently, a group led by Prof. XIAO jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reviewed an innovate scheme, namely reaction phase diagram (RPD) for computational design of chemical reactions. It can offer not only an in-depth understanding of reaction mechanisms, but also the prediction of catalytic activity and selectivity trend over a collection of catalysts.
This study was published in WIREs Comput. Mol. Sci. on Jan. 5.
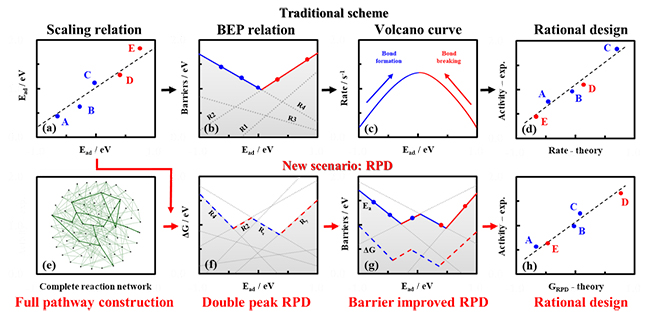
Figure: Illustration of traditional strategy (black arrow) and innovate reaction phase diagram (red arrow) for catalyst design (Image by GUO Chenxi)
The theoretical catalyst design of RPD started from a "full pathway construction", where all possible reaction pathways could be enumerated based on a set of elementary steps. Different optimal mechanisms could be obtained following the global optimization considering all possible pathways at given descriptors, and the activity optimums could be derived due to diverse reaction pathways.
"We could obtain more accurate activity trends based on the GRPD-limiting energies with kinetic calculations, and it could result in more reasonable analysis of mechanism and catalyst design," said Prof. XIAO.
The RPD analysis with the scheme of reaction global optimizations was applied to understand various catalytic processes, including the double-peak activity trend for CO2 electroreduction, the potential dependent activity trend for oxygen reduction reaction (ORR), and the complex selectivity analysis in CO conversion producing C2 products.
Moreover, it provided a successful case of catalyst rational design with a target of NO selective electroreduction to ammonia.
This study was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the Liaoning Revitalization Talents Program. (Text by GUO Chenxi)