Scientists from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and Peking University revealed ordered to disordered transformation of enhanced water structure on hydrophobic surfaces in concentrated alcohol-water solutions. The research was published in Journal of Physical Chemistry Letters.
The effects of hydrophobic solutes on the structure of the surrounding water have been a topic of debate for almost 70 years. However, a consistent description of the physical insight into the causes of the anomalous thermodynamic properties of alcohol-water mixtures is lacking.
This work employed the temperature-dependent FTIR spectroscopy combined with the femtosecond infrared spectroscopy to explore the water structural transformation in concentrated alcohol-water solutions.
Experiments show that the enhancement of water structure arises around micro-hydrophobic interfaces at room temperature in the solutions. As temperature increases, this ordered water structure disappears and a surface topography dependent new disordered water structure arises at concentrated solutions of large alcohols. A more-ordered-than-water structure can transform into a less-ordered-than-water structure.
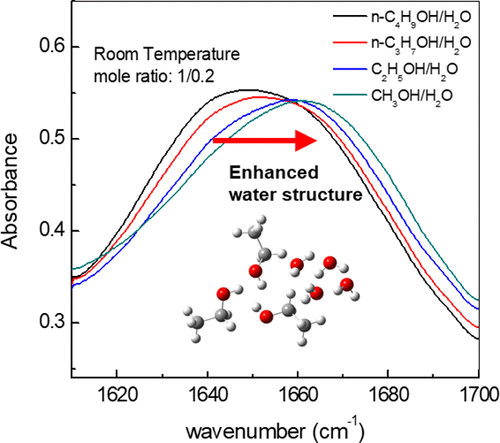
Effect of alcohol size on water structural transformation in concentrated alcohol-water solutions. (Image by YUAN Kaijun)
This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the Chemical Dynamics Research Center. (Text by YUAN Kaijun)