A research team led by Prof. DAI Wen from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. ZHANG Zehui from South-Central University for Nationalities, developed a new strategy for heterogeneous catalytic successive cleavage and functionalization of C–C bonds in alcohols.
Their findings were published in Chem on Mar. 25.
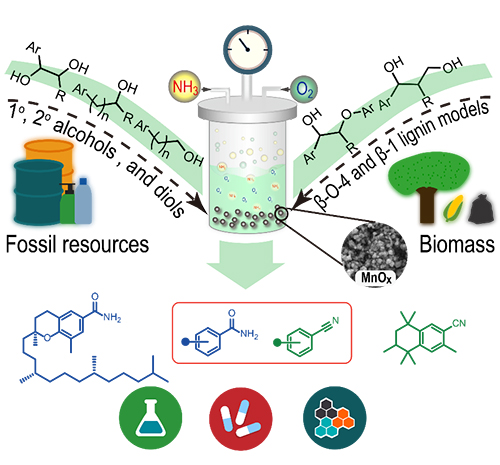
Schematic illustrations of the manganese oxide for catalytic cleavage and functionalization of alcohols reaction (Image by HE Peipei)
Given that alcohols can be broadly accessible from both fossil resources and naturally renewable biomass, the oxidative cleavage and functionalization of C–C bonds in alcohols has emerged as a powerful tool for the conversion of alcohols to a variety of value-added chemicals.
Indeed, in the past decades, some homogeneous catalytic systems have been well established for the cleavage and functionalization of alcohols. However, over-reliance on stoichiometric and environmentally unfriendly oxidants, a large excess of a strong base, and/or high loading of precious metal catalysts still limit most of these reaction classes.
The researchers developed a novel and efficient protocol that enables the direct synthesis of amides via heterogeneous manganese oxide catalyzed successive cleavage and amidation of C-C bonds in alcohols with oxygen as an environmentally benign oxidant and ammonia as nitrogen source.
They found that a wide range of primary and secondary alcohols, 1,2-diols, and even β-O-4 and β-1 lignin model compounds could undergo C–C bond cleavage smoothly to deliver one- or multiple-carbon shorter amides. Moreover, a slight modification of the reaction conditions allowed for the cleavage and cyanation of alcohols to access sterically hindered nitriles.
This protocol features good functional-group tolerance, facile scalability, a cost-effective and recyclable catalyst, the use of readily available starting materials, a broad substrate scope. It has been applied to the late-stage amidation and cyanation of bioactive alcohols, which offers an efficient way to generate natural product derivatives for drug discovery and chemical biology.
"Therefore, this method not only complements existing protocols for transformations of alcohols but also expands the new application of the abundant fossil resources and renewable biomass," said Prof. DAI.
The above research was supported by DICP and the National Natural Science Foundation of China. (Text by HE Peipei)