The catalytic activation and the transformation of dinitrogen to ammonia under mild condition is one of the greatest challenges in chemistry. And it has been actively investigated for a century. The research groups led by Prof. JIANG Ling and Prof. CHEN Ping from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) collaboratively process an important progress on mechanism understanding of catallytic ammonia synthesis. The results of this research have been published in Angew. Chem. Int. Ed. entitled as "The formation of surface lithium–iron rernary hydride and its function on catalytic ammonia synthesis at low temperatures." (DOI: 10.1002/anie.201703695).
Their experimental results show that the Li-Fe ternary hydride species (such as Li4FeH6 and Li5FeH6) at the surface/interface of the catalyst are existing. These ternary hydrides can react with N2 to form –NH2 contained clusters (e.g. Fe-(NH2)-Li and LiNH2). This reaction proves that N≡N bond can be facilely broken and the activated N can be hydrogenated to -NH2 by hydridic H in the Li-Fe hydride. Therefore, they deduce that those ternary hydride species may serve as active center for ammonia synthesis over the LiH-Fe composite catalyst. And the N2 activation and hydrogenation are performed on the Lin[FeH6] complex rather than on the C7 site of conventional Fe-based catalyst.
With further experimental and theoretical studies, a better understanding would be achieved which are beneficial for the design of catalyst and the development of energy-efficient ammonia synthesis. Such advances may be integrated into clean and renewable energy-harvesting and storage technology.
Previously, the DICP research group led by Prof. CHEN Ping developed a composite catalyst system, which is made of LiH and 3d metals (such as Fe). This system can catalyze the formation of ammonia from N2 and H2 at 150 °C and can significantly outperform the most active catalysts to date (Nature Chemistry, 9 (2017) 64). Perceptively, this composite catalyst system presents distinct features from conventionally available catalysts such as Fe- or Ru- based catalysts. However, the molecule-level understanding of the nature of active site, the activation of N2 and the formation of NH3 of this new-developed catalyst system are needed to be further investigated.
The DICP research group led by Prof. JIANG Ling has strong expertise in gas-phase optical spectroscopy coupled with mass spectrometry. This technique is used to successfully identify and analyze the clusters that may correlate well with the active species on catalyst surface.
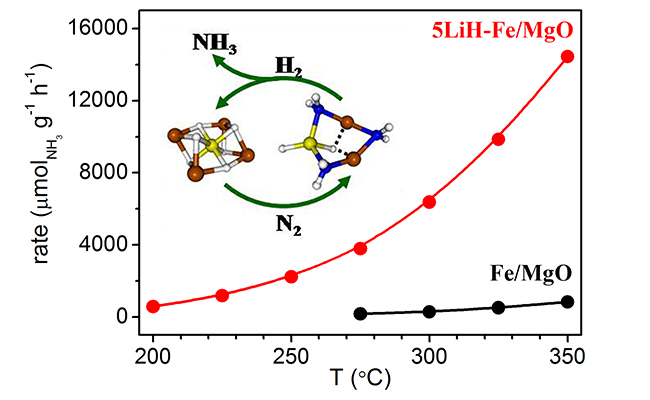
The formation of Li4FeH6 and its function on catalytic ammonia synthesis (Image by WANG Peikun and XIE Hua)
This work was financially supported by National Science Funds for Distinguished Young Scholars, National Natural Science Foundation of China, the Collaborative Innovation Center of Chemistry for Energy Materials (2011-iChEM) and Dalian Institute of Chemical Physics (DICP DMTO201504). (Text and Image by JIANG Ling, WANG Peikun and XIE Hua)
Dr. WANG Yongjin
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn