Recently, a research team led by Prof. CHEN Ping and Dr. GUO Jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and their collaborators developed high efficiency manganese-based catalysts for ammonia synthesis.
This research entitled "Alkali and alkaline earth hydrides-driven N2activation and transformation over Mn nitride catalyst" was published in J. Am. Chem. Soc. (DOI: 10.1021/jacs.8b08334).
Ammonia synthesis over transition metals is very important in heterogeneous catalysis. Iron and ruthenium have moderate nitrogen adsorption energies, and therefore exhibit excellent ammonia synthesis performances and have been used extensively in industry.
Early transition metals such as V, Cr, and Mn, however, own strong nitrogen adsorption capability and usually form stable nitride phases under the reaction conditions of ammonia synthesis, which hinders the sequent hydrogenation steps and thus showing poor catalytic activities.
Based on previous research on ammonia synthesis, the scientists investigated the influence of alkali or alkaline earth metal hydrides (AH) on the catalytic performance of Mn-based catalysts.
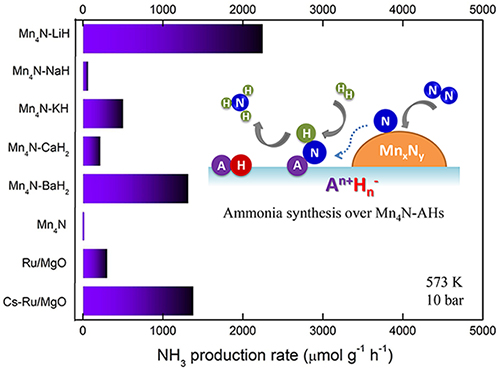
Alkali or alkaline earth metal hydrides activate manganese nitride in ammonia synthesis catalysis. (Image by CHANG Fei)
Experimental results indicated that the catalytic activities of Mn-AH composites were 1-3 orders of magnitude higher than that of Mn catalyst itself. Among them, the performances of Mn-LiH and Mn-BaH2 were comparable to that of Ru-based catalyst.
The order of promotion effect of AH for Mn was different from that of conventional alkali or alkaline earth (hydr)oxide electronic promoters. Thermodynamic analyses and characterization results revealed that the phase transformation between alkali or alkaline earth metal hydrides and their imides, as well as their interactions with Mn nitride under the reaction conditions of ammonia synthesis should account for the promotion effect.
More detailed investigation on the activity-phase structure relationship of Mn-LiH catalyst indicated the active phases and kinetic behaviors of these composite catalysts strongly depended on the reaction conditions especially temperature and space velocity. This was another unique feature of these composite catalysts that was different from the conventional ammonia synthesis catalysts.
The research proposes a promising strategy for “activating” the early transition metals in ammonia synthesis catalysis.
This study was conducted in collaboration with Prof. LI Xingguo from Peking University. And it was supported by the National Natural Science Foundation of China, Sino-Japanese Research Cooperative Program of Ministry of Science and Technology, Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), DICP DMTO, and Youth Innovation Promotion Association of Chinese Academy of Sciences. (Text by CHANG Fei and GUO Jianping)