The freezing of water is one of the most common everyday processes. However, understanding the microstructure of ice and its hydrogen-bonding networks has been a challenge.
The low-energy structures of water octamer are predicted to be nominally cubic, with the eight tri-coordinated water molecules at the eight corners of the cube. Such tri-coordinated water molecules have been identified at the surface of ice.
Only a few gas-phase studies have been achieved for experimental characterization of water octamer, and two nearly isoenergetic structures with D2d and S4 symmetry are found.
This understanding now has changed.A research team led by Prof. JIANG Ling and Prof. YANG Xueming from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. LI Jun from Tsinghua University, revealed the coexistence of five cubic isomers in the smallest ince cube, including two with chirality.
The study was published in Nature Communications on October 28.
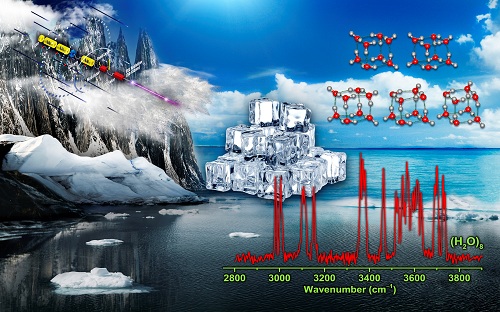
Scientists discovered new structures in smaller ice cube (Image by LI Gang and LI Qinming)
Prof. JIANG and Prof. YANG developed a method of infrared spectroscopy of neutral clusters based on a tunable vacuum ultraviolet free electron laser (VUV-FEL). This method created a new paradigm for the study of vibrational spectra of a wide variety of neutral clusters that could not be studied before.
"We measured infrared spectra of size-selected neutral water octamer using the VUV-FEL-based infrared scheme" said Prof. JIANG.
"We observed the distinct features in the spectra, and identified additional cubic isomers with C2 and Ci symmetry, which coexisted with the global-minimum D2d and S4 isomers at finite temperature of the experiment," said Prof. YANG.
Prof. LI's team conducted quantum chemical studies to understand the electronic structure of the water octamer. They found that the relative energies of these structures reflect topology-dependent, delocalized multi-center hydrogen-bonding interactions.
The study demonstrated that even with a common structural motif, the degree of cooperativity among the hydrogen-bonding network created a hierarchy of distinct species. It provided crucial information for fundamental understanding of the formation processes of cloud, aerosol, and ice, especially under rapid cooling.
Their findings provide a benchmark for accurate description of the water intermolecular potentials to understand the macroscopic properties of water and stimulate further study of intermediate-ice structures formed in the crystallization process of ice. (Text by LI Gang and LI Qinming)