Research News
-
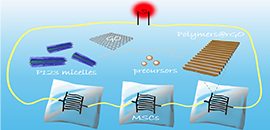 06 10, 2019Two-Dimensional Polymers with Cylindrical Mesopores on Graphene for Micro-Supercapacitors DevelopedScientist developed 2D polymers with in-plane cylindrical mesopores on graphene nanosheets for micro-supercapacitors.
06 10, 2019Two-Dimensional Polymers with Cylindrical Mesopores on Graphene for Micro-Supercapacitors DevelopedScientist developed 2D polymers with in-plane cylindrical mesopores on graphene nanosheets for micro-supercapacitors.
A research group led by Prof. WU Zhongshuai from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with MAI Yiyong from Shanghai Jiao Tong University, developed 2D polymers with in-plane cylindrical mesopores on graphene nanosheets for micro-supercapacitors. The study was published in Angew. Chem. Int. Ed..
Two-dimensional (2D) nanomaterials, such as graphene, exhibit unique properties absent in their bulk materials. However, these 2D nanosheets easily tend to stack or aggregate together, leading to the limited accessibility of active sites and thus unsatisfied performance in energy-related applications, e.g., micro-supercapacitors (MSCs).
The growth of electrochemically active moieties on 2D nanosheets to produce 2D sandwich-like heterostructures was proven to be a promising strategy for the prevention of the stacking and thus the improvement of their capacitive performance in MSCs. On the other hand, mesoporous structure in electrode materials can largely increase specific surface area, reserve the electrolyte and facilitate the ionic diffusion pathways.
Thereby, patterning well-defined mesoporous electrochemically active materials on free-standing nanosheets, such as graphene, will produce novel 2D sandwich-structured hybrid nanomaterials for high-performance MSCs. Nevertheless, this remains a great challenge lacking of suitable templates and innovative techniques.
Schematic of the fabrication of 2D mesoporous polymer/graphene with in-plane cylindrical pores for MSCs; Morphological characterizations of mesoporous polypyrrole/graphene nanosheets; Schematic diagram of the roles of cylindrical mesopores parallel to graphene surface in the high performance of planar MSCs. (Image by QIN Jieqiong and HOU Dan)
To address the above issue, the scientists synthesized polypyrrole, polyaniline and polydopamine with in-plane cylindrical mesopores on graphene nanosheets by the interface self-assembly strategy.
The resultant 2D sandwich-structured nanohybrids were employed as the interdigital microelectrodes for the assembly of planar MSCs, which delivered outstanding volumetric capacitance and energy density. Moreover, the MSCs displayed remarkable flexibility and superior integration for boosting output voltage and capacitance.
The interfacial self-assembly protocol opens novel opportunities for patterning 2D porous nanosheets for planar energy storage devices.
The above work was supported by China's National Natural Science Foundation, and China's National Key R&D Program, etc. (Text by QIN Jieqiong) -
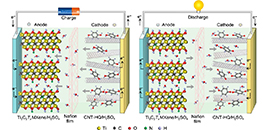 06 06, 2019Scientists Develop High-Energy-Density Hydrogen-Ion-Rocking-Chair SupercapacitorsScientists developed high-energy-density, hydrogen-ion-rocking-chair MXene based hybrid supercapacitors. The finding was published in ACS Nano.
06 06, 2019Scientists Develop High-Energy-Density Hydrogen-Ion-Rocking-Chair SupercapacitorsScientists developed high-energy-density, hydrogen-ion-rocking-chair MXene based hybrid supercapacitors. The finding was published in ACS Nano.
A research group led by WU Zhong-Shuai from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences, in collaboration with WANG Xiaohui from the Institute of Metal Research of the CAS, developed high-energy-density, hydrogen-ion-rocking-chair MXene based hybrid supercapacitors. The study was published in ACS Nano.
MXene, a family of two-dimensional metal carbides and carbonitrides, have attracted widespread interest due to their excellent electrochemical energy storage properties. As a representative, Ti3C2Tx has already demonstrated to be a promising pseudocapacitive electrode material with high capacitance of 1500 F/cm3 (380 F/g).
However, aqueous symmetric Ti3C2Tx-based supercapacitors are usually limited by low voltage window (~0.6 V), due to irreversible oxidation of Ti3C2Tx at high potential. Hence, integrating high-capacitance, high-conductivity and electrochemically stable cathode and MXene anode is necessary to build high-voltage, high-energy-density MXene-based supercapacitors.
Schematic of the hydrogen-ion-rocking-chair MXene carbon nanotube-hydroquinone hybrid supercapacitors. (Image by SHI Xiaoyu and HOU Xiaocheng)
Taking Ti3C2Tx as negative electrode, carbon nanotubes as positive electrode, H2SO4 solutions as negative electrolyte, H2SO4 solutions containing hydroquinone as positive electrolyte, and a proton-permeable Nafion film as separator, the researchers fabricated asymmetric hybrid supercapacitors.
Hydroquinone and quinone reversibly converted to each other during charge-discharge, accompanied by protonation and deprotonation processes. Meanwhile, hydrogen ions reversibly intercalated and extracted in Ti3C2Tx MXene anode, inducing a change of the titanium oxidation state.
This reversible electrochemical behavior was accompanied by hydrogen ion fast transport between the positive and negative electrodes, thereby the device was called as hydrogen-ion-rocking-chair hybrid supercapacitors, similar to lithium-ion batteries.
Attributed to high pseudo capacitance of both electrodes, matched potential window and excellent reversibility, the hybrid supercapacitors exhibited a voltage up to 1.6 V, significant stability of 100% retention after 5000 cycles and a record energy density of 62 Wh/kg, exceeding all reported aqueous MXene-based supercapacitors.
This work will advance high-energy-density electrochemical energy storage devices toward actual applications.
The above research was supported by National Natural Science Foundation of China, National Key R&D Program of China etc. (Text by SHI Xiaoyu and HOU Xiaocheng) -
 06 03, 2019CHEN Ping Wins "Innovation Mission Champions"
06 03, 2019CHEN Ping Wins "Innovation Mission Champions"
The Tenth Clean Energy Ministerial (CEM-10) and The Fourth Mission Innovation Ministerial (MI-4) were successfully held on May 27 to 29, 2019 in Vancouver, Canada.
Dr. CHEN Ping, the Professor of Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, was awarded as “Mission Innovation Champions” for her pioneer research in hydrogen storage materials.
Mission Innovation Programme launched at the Paris Climate Conference in 2015 by 22 countries and the European Commission seeking to dramatically accelerate global clean energy innovation and promotion. As part of the global MI Programme, the Mission Innovation Champions Programme launched in 2018 seeks to celebrate and support innovative individuals who are accelerating the clean energy revolution.
Following a rigorous competition, including peer-to-peer reviews and evaluations by a diverse panel of experts from around the globe, 19 Champions working in a variety of energy-related fields and industries were awarded, Dr. CHEN Ping is the only Chinese scholar received this honor. (Text by XIE Dong and HE Teng) -
 06 03, 2019Prof. J.A.M. Kuipers Presents the XXXII Zhang Dayu Lecture
06 03, 2019Prof. J.A.M. Kuipers Presents the XXXII Zhang Dayu Lecture
Prof. J.A.M. Kuipers from Eindhoven University of Technology, the Netherlands, presented the XXXII Zhang Dayu lecture entitled “Recent advances in the multi-scale simulation of mass, momentum and heat transfer in multiphase chemical reactors” at the Conference Room of Energy Fundamentals Building on Friday May 24th, 2019.
Prof. YE Mao chaired the lecture, and Prof. CAI Rui, the deputy director of DICP, awarded Prof. Kuipers the “Zhang Dayu Lectureship” certification and granted him the Honored Professorship of DICP. More than 100 staffs and students with relevant research background attended this event.
The research interests of Prof. Kuipers included the multi-scale modelling and advanced measurement technologies of multiphase flows in chemical reactor. He was the first person who published more than 100 papers in Chemical Engineering Science in 2013.
Prof. Kuipers was elected to the Royal Netherlands Academy of Arts and Sciences (KAWN) in 2015. In his talk, Prof. Kuipers introduced the recent advances in the multi-scale modelling of mass, momentum and heat transfer in multiphase chemical reactors. He also answered many questions from the audiences and discussed on some specific issues with DICP young scientists.
During his stay in DICP, Prof. Kuipers visited the National Engineering Laboratory for Methanol to Olefins, Laboratory of Catalysts and New Materials for Aerospace, and Laboratory of Microchemical Engineering and Technology.
Prof. Zhongmin Liu, the director of DICP, also met Prof. Kuipers after the lecture, and discussed the possibility of the further collaborations in multiphase reaction engineering. (Text by YE Mao and LIU Jiajia) -
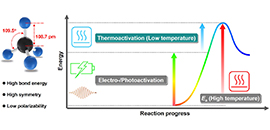 06 03, 2019Direct Methane Conversion under Mild Conditions by Thermo-, Electro- or Photocatalysis Reviewed
06 03, 2019Direct Methane Conversion under Mild Conditions by Thermo-, Electro- or Photocatalysis Reviewed
Direct conversion of Earth-abundant methane into value-added chemicals under mild conditions is an attractive technology in response to the increasing industrial demand for feedstocks and the worldwide appeal of energy conservation. Exploring advanced low-temperature C-H activation catalysts and reaction systems is the key to converting methane in a direct and mild manner.
Recently, a research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences reviewed the latest progress in low-temperature methane conversion in thermocatalytic, electrocatalytic, and photocatalytic systems. The study was published in Chem.
"We summarized the typical catalysts employed in various reaction systems, especially the heterogeneous catalysts with noteworthy C-H activation performance," said Prof. DENG.
Molecular structure of methane (Left) and energy diagram for methane activation in electro-/photoactivation involved reaction systems (Right).(Image by MENG Xianguang and GAO Hehua)
"The viewpoints on the catalyst design, theoretical simulations, choice of reaction conditions, and method of reaction product analysis were introduced to encourage more viable technology for low-temperature methane conversion in the future," said Prof. DENG.
The researchers also pointed out the importance of coupling multiple driving forces from thermal, electrical and solar energy to jointly activate methane by integrating the advantages of these activation pathways in one reaction system.
Prof. DENG's group has been focusing on the development of 2D material-based catalysts and their applications in the catalytic conversion of energy-related molecules (Nature Nanotechnology, 2016, 11, 218-230; Chemical Reviews, 2019, 119, 1806-1854).
As early as 2015, Prof. DENG and Prof. BAO Xinghe, et al. reported the capability of graphene-confined single iron sites for the catalytic oxidation of complicated hydrocarbons at room temperature (Science Advances, 2015, 1, e1500462).
Notable recent progress by the group includes the finding that graphene-confined single iron atoms could even catalyze methane conversion at room temperature (Chem, 2018, 4, 1902-1910).
These results demonstrate bright prospects for 2D-based catalysts in the application of C-H activation and other useful catalytic processes.(Text by MENG Xianguang and GAO Hehua) -
 05 29, 2019DICP Scientists Achieve High-Quality Gasoline Directly from SyngasScientists achieved directly synthesis of high-quality gasoline from syngas.Recently, a research group led by Profs. PAN Xiulian and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences achieved directly synthesis of high-quality gasoline from syngas, their findings were published in Angewandte Chemie International Edition.Schematic of high-quality gasoline synthesis directly from syngas over OX-ZEO bifunctional catalysts. (Image by JIAO Feng)Syngas is an important intermediate platform for efficient utilization of carbon resources such as coal, natural gas and biomass, which can be converted to a variety of chemicals and fuels. However, precise control C-C coupling to fabricate desired products remains a challenge in syngas chemistry.The OX-ZEO (Oxide-Zeolite) catalyst design concept, which was reported by this research group, has enabled selective syngas conversion to mixed light olefins with a high selectivity of 80% among hydrocarbons, to ethylene with a selectivity of 83%, and to aromatics with a selectivity of 73.9% , far beyond the limits predicted by the ASF distribution model.“Here we reported one step transformation of syngas to gasoline with a selectivity of 77% at CO conversion of 20%. The hazardous aromatics content in gasoline (< 16%) was significantly lower than the limits set by most countries.” said Prof. PAN.“The ratio of isoparaffins to n-paraffins was as high as 15, giving a research octane number of 92 in contrast to about 35~43 obtained by the conventional low temperature Fischer-Tropsch synthesis.” PAN added.The aboved research provided a potential technology for one-step synthesis of high quality gasoline from a variety of carbon resources via syngas. It demonstrated again that the OX-ZEO catalyst concept was general and could be applicable for synthesis of other chemicals from syngas.This work was supported by the Ministry of Science and Technology of China, the Chinese Academy of Sciences and the National Natural Science Foundation of China. (Text by LI Na)
05 29, 2019DICP Scientists Achieve High-Quality Gasoline Directly from SyngasScientists achieved directly synthesis of high-quality gasoline from syngas.Recently, a research group led by Profs. PAN Xiulian and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences achieved directly synthesis of high-quality gasoline from syngas, their findings were published in Angewandte Chemie International Edition.Schematic of high-quality gasoline synthesis directly from syngas over OX-ZEO bifunctional catalysts. (Image by JIAO Feng)Syngas is an important intermediate platform for efficient utilization of carbon resources such as coal, natural gas and biomass, which can be converted to a variety of chemicals and fuels. However, precise control C-C coupling to fabricate desired products remains a challenge in syngas chemistry.The OX-ZEO (Oxide-Zeolite) catalyst design concept, which was reported by this research group, has enabled selective syngas conversion to mixed light olefins with a high selectivity of 80% among hydrocarbons, to ethylene with a selectivity of 83%, and to aromatics with a selectivity of 73.9% , far beyond the limits predicted by the ASF distribution model.“Here we reported one step transformation of syngas to gasoline with a selectivity of 77% at CO conversion of 20%. The hazardous aromatics content in gasoline (< 16%) was significantly lower than the limits set by most countries.” said Prof. PAN.“The ratio of isoparaffins to n-paraffins was as high as 15, giving a research octane number of 92 in contrast to about 35~43 obtained by the conventional low temperature Fischer-Tropsch synthesis.” PAN added.The aboved research provided a potential technology for one-step synthesis of high quality gasoline from a variety of carbon resources via syngas. It demonstrated again that the OX-ZEO catalyst concept was general and could be applicable for synthesis of other chemicals from syngas.This work was supported by the Ministry of Science and Technology of China, the Chinese Academy of Sciences and the National Natural Science Foundation of China. (Text by LI Na)