Research News
-
 01 24, 2022Multi-organoid System to Simulate Human liver-islet Axis in Normal and Type 2 DiabetesScientists developed a multi-organoid system from human induced pluripotent stem cells (hiPSCs) to recapitulate human liver-islet axis in normal and type 2 diabetes.
01 24, 2022Multi-organoid System to Simulate Human liver-islet Axis in Normal and Type 2 DiabetesScientists developed a multi-organoid system from human induced pluripotent stem cells (hiPSCs) to recapitulate human liver-islet axis in normal and type 2 diabetes.
Type 2 diabetes (T2DM) is a systematic multi-organ metabolic disease, which is characterized by dynamic interplay among different organs.
Pancreatic islet-liver axis is closely associated with normal glucose regulation and homeostasis maintenance. The dysfunction of the interplay between these organs may lead to T2DM progression.
Recently, a group led by Prof. QIN Jianhua from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a multi-organoid system from human induced pluripotent stem cells (hiPSCs) to simulate human liver-islet axis in normal and T2DM.
This study was published in Advanced Science on Dec. 23, 2021.
"Stem cell-derived organoid, a new class of 3D tissues, has the key structural and functional features of in vivo counterparts," said Prof. QIN.
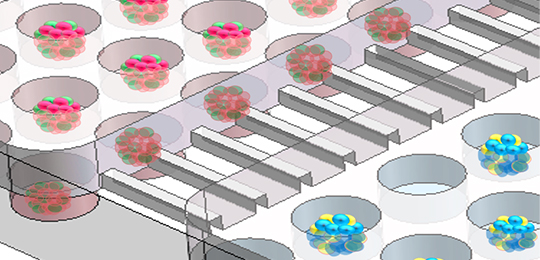
This newly developed multi-organoid system could simulate human liver-pancreatic islet axis in physiological and pathological condition, and enable 3D co-culture of hiPSC-derived liver and islet organoids for up to 30 days.
The researchers found that the generated liver and islet organoids exhibited favorable growth and improved tissue-specific functions in this perfused microfluidic 3D culture system.
"The sensitive glucose-stimulated insulin secretion was from the islet organoids and it increased glucose utilization in the liver organoids," said Prof. QIN. This reflected the cooperative interaction between the two organs in this perfused co-culture system.
Transcriptional analyses revealed that the activated signaling pathways were associated with glucose/CYP450 metabolism and glycolysis/gluconeogenesis in liver and islet organoids.
What's more, this microfluidic islet-liver organoid system exhibited mitochondrial dysfunction and decreased glucose transport capacity under hyperglycemic condition, which might be attributed to the alleviated by treatment with anti-diabetic drug.
Schematic of hiPSCs derived multi-organoid-on-chip system to model human liver-pancreatic islet axis in vitro (Image by TAO Tingting)
"This novel system can not only reflect the feedback loop within the liver-islet axis, but also exhibit relevant responses to high glucose and anti-diabetes drug, which is not easily achieved by conventional cell culture and animal models," said Prof. QIN. "It will provide a unique platform for the study of complex T2DM pathogenesis and drug development."
This research was supported by the National Key R&D Program of China and the Strategic Priority Research Program of CAS. (Text by TAO Tingting) -
 01 21, 2022Germanium Halides Serve as Ideal Precursors to Synthesize High-quality Perovskite NanocrystalsScientists served all-inorganic nonhazardous GeX4 (X = Cl, Br, I) as ideal halide sources to synthesize both Pb-free and Pb-based PNCs with high-optoelectronic-quality.
01 21, 2022Germanium Halides Serve as Ideal Precursors to Synthesize High-quality Perovskite NanocrystalsScientists served all-inorganic nonhazardous GeX4 (X = Cl, Br, I) as ideal halide sources to synthesize both Pb-free and Pb-based PNCs with high-optoelectronic-quality.
Organohalides are widely adopted to serve as halide source in traditional three-precursors route to obtain metal halide perovskite nanocrystals (PNCs).
However, these organohalides are usually highly toxic, which is unfavorable for large-scale and sustainable use. Moreover, their efficacy in producing high-quality Pb-free PNCs is questionable.
Recently, a research group led by Prof. HAN Keli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) used all-inorganic nonhazardous GeX4 (X = Cl, Br, I) as ideal halide sources to synthesize both Pb-free and Pb-based PNCs with high optoelectronic quality.
This study was published in Nano Letters on January 12.
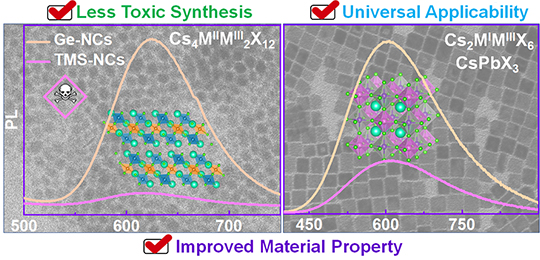
Germanium halides as ideal precursors for less toxic synthesis of high optoelectronic quality metal halide perovskite nanocrystals (Image by WANG Xiaochen)
They found that Ge element wasn't present in the final compositions, whereas material properties of the resulting NCs were improved, such as the stronger photoluminescence emission and enhanced phase stability.
They attributed these improved properties to a better control over the release of halide ions in Ge halide-based route, which helped the PNCs to form a regular crystal surface with less point defects.
Moreover, the advantage of the proposed GeX4 approach in making ideal PNCs lied in their unique dielectric environment and thermodynamics.
"It is foreseen that the GeX4-based synthesis could provide a yet unexplored less toxic path to produce these fascinating nanomaterials and tailor their optical properties without alerting their intrinsic structure," said Prof HAN.
This work was supported by the National Natural Science Foundation of China. (Text by WANG Xiaochen) -
 01 18, 2022Hetero-Lattice Intergrown and Robust MOF Membranes: A Potential Solution to Polyol Upgrading in Industryproposed hetero-lattice intergrown (HLI) and robust MOF membranes, which would be a potential solution to polyol upgrading in industry.
01 18, 2022Hetero-Lattice Intergrown and Robust MOF Membranes: A Potential Solution to Polyol Upgrading in Industryproposed hetero-lattice intergrown (HLI) and robust MOF membranes, which would be a potential solution to polyol upgrading in industry.
Metal-organic frameworks (MOFs) bring tremendous opportunities for separation of liquid chemicals using membranes.
However, due to lack of highly compact and robust micro-architecture to cope with complicated and tough separation situations, MOF membranes available for liquid chemical upgrading through pervaporation are rare.
Recently, a research group led by Prof. YANG Weishen and Dr. BAN Yujie from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed hetero-lattice intergrown (HLI) and robust MOF membranes for polyol upgrading in industry.
This study was published in Angew. Chem. Int. Ed. on Dec. 22, 2021, and was selected as "Hot Paper".
Pervaporation performances through the optimal HLI membranes (Image by BAN Yujie and WANG Yuecheng)
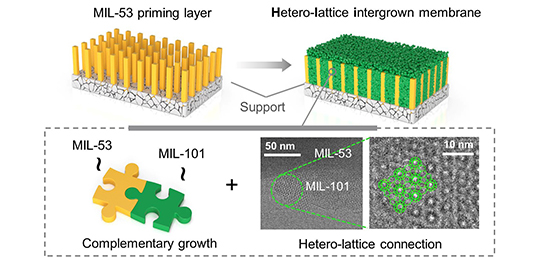
The researchers synthesized HLI membranes, with the integration of two distinct network MOFs, namely MIL-53 (Al) and amino-MIL-101 (Cr), at molecular scale.
They demonstrated that the highly compact and robust micro-architecture was contributed to the complementary growth, concomitant with the strong connection between these two lattices.
The HLI membrane with ultra-stability showed excellent pervaporation dehydration performances for C2-C4 polyol solutions. Furthermore, polymer-grade ethanediol (99.93%) through HLI membranes could be obtained, saving ca. 32% of energy consumption relative to the traditional vacuum distillation.
"These results spotlight the potential of MOF membranes to create more solutions for current separation challenges," said Prof. YANG.
This work was supported by the National Natural Science Foundation of China. (Text by BAN Yujie and WANG Yuecheng) -
 01 12, 2022New Catalytic Approach Directly Converts Raw Biomass into Natural Gas with Low Carbon Footprint
01 12, 2022New Catalytic Approach Directly Converts Raw Biomass into Natural Gas with Low Carbon Footprint
Natural gas can be used as fuel for generating electricity, heating and powering transportation. It is also the raw material to manufacture hydrogen and ammonia.
Currently, biogenic and thermogenic processes are the two widely accepted methods for natural gas production. Whereas, high content of CO2 in gas products is inevitable during these processes, which cannot satisfy the composition requirements of pipeline natural gas.
A research group led by Prof. LU Fang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed an efficient catalytic approach to directly transfer solid biomass into natural gas with low carbon footprint.
This study was published in Nature Communications on January 11.
The researchers prepared a robust catalyst with Ni2Al3 alloy phase, achieving nearly complete conversion of various agricultural and forestry to natural gas. In the conversion process, the total carbon yield of gas products reached up to 93% after several hours, and the catalyst showed powerful processing capability for the production of natural gas during 30 cycles.
"Life cycle assessment revealed that the life cycle primary fossil energy depletion and greenhouse gas emissions in this process could be reduced by 26% and 34%, respectively, compared to the fossil-natural gas," said Prof. LU.
Conceptual diagram for bio-natural gas production from biomass (Image by SI Xiaoqin)
What's more, bio-natural gas produced from the combined hydrogen with lignocellulosic biomass can be further applied in industry, transportation, and electric power plant by the existing transportation pipelines.
This study provides a new guidance for the catalytic transformation of raw biomass. It was supported by the Natural Science Foundation of China, the National Key R&D Program of China, the Strategic Priority Research Program of CAS, and DICP Grant. (Text by SI Xiaoqin) -
 12 29, 2021Scientists Discover Simple and Effective Model to Predict Noble Metal Catalyst StructuresScientists provided a simple and effective mode for predicting the metal surfaces involved in catalytic process.
12 29, 2021Scientists Discover Simple and Effective Model to Predict Noble Metal Catalyst StructuresScientists provided a simple and effective mode for predicting the metal surfaces involved in catalytic process.
Noble metals have incomparable advantages as special catalysts.
However, due to the influence of the relativistic effect, it's difficult to explain the specific structure and reaction mechanism of noble metals catalysts.
Recently, a research group led by Prof. JIANG Ling and Assoc. Prof. XIE Hua from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Assoc. Prof. LIU Zhiling's group from Shaanxi Normal University, provided a simple and effective mode for predicting the metal surfaces involved in catalytic process.
This study was published in The Journal of Physical Chemistry Letters on Dec. 23.
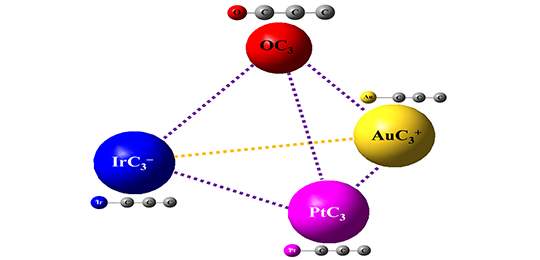
Isoelectronic IrC3–, PtC3 and AuC3+ Clusters Featuring the Structural and Bonding Resemblance to OC3 (Image by LIU Xuegang and XIE Hua)
The researchers investigated IrC3–, PtC3 clusters using photoelectron spectroscopy and density functional theory calculations.
They revealed that the IrC3–, PtC3, AuC3+ and OC3 complexes had analogous chemical bonding properties, which indicated that the metal atom could be viewed as oxygen atom in the isoelectronic species (IrC3–, PtC3, AuC3+) systems.
"Our finding broadened the notion of autogenic isolobality to the gas phase clusters that contain Ir–, Pt, Au+ and C centers," said Prof JIANG. "It provides a simple and effective empirical model for exploring the chemistry of noble mental by gas-phase chemistry," said Prof. JIANG.
This work was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of CAS, the Strategic Priority Research Program, International Partnership Program, and K. C. Wong Education Foundation. (Text by LIU Xuegang and XIE Hua) -
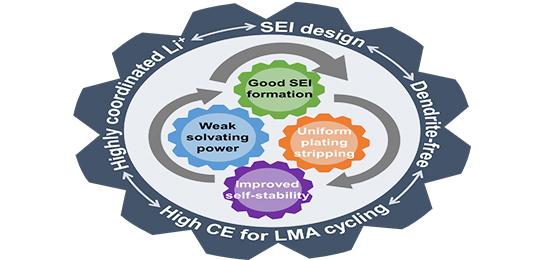 12 27, 2021Dual Strategy with Li-ion Solvation and Solid Electrolyte Interphase Improves Stability of Lithium Metal AnodeScientists developed a dual-strategy with Li-ion solvation and SEI regulation to stabilize lithium metal anode.
12 27, 2021Dual Strategy with Li-ion Solvation and Solid Electrolyte Interphase Improves Stability of Lithium Metal AnodeScientists developed a dual-strategy with Li-ion solvation and SEI regulation to stabilize lithium metal anode.
Lithium metal is an ideal anode for high-energy-density rechargeable batteries due to its lowest electrochemical potential and high theoretical capacity.
However, the cycling of lithium metal anode (LMA) is limited by the uneven Li plating-stripping and unstable solid electrolyte interphase (SEI).
Recently, a research group led by Prof. CHEN Jian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a dual-strategy with Li-ion solvation and SEI regulation to stabilize lithium metal anode.
This study was published in Energy Storage Materials on Oct. 8.
Dual strategy for improving LMA Coulombic efficiency (Image by ZHU Shengdong)
The researchers used 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) as the solvent, separately blending with fluorinated ether (TTE), to investigate the effect of the solvents for their solvation and electrochemistry in localized high concentration electrolytes (LHCEs).
They found that the weakly solvating capability of DOL weakened the Li-ion interaction with solvents but improved the coordination capability with anions. Hence, DOL-TTE formed a unique solvation structure that differed from DME-TTE blends.
A small amount of TTE could dramatically reshape the coordination structure of the Li-ion in DOL while it could not work in DME. With increasing TTE additions in DOL-TTE blends, ionic aggregates became the dominant solvating configuration, facilitating more anion-derived SEI and dendrites-free morphology.
Moreover, the highly coordinated Li+ solvation of DOL-TTE (1:2) electrolyte could limit the dissolution and loss of LiPSs in Li-SPAN cells, obtaining a high Coulombic efficiency of 99.77% and capacity retention of 92% after 100 cycles.
"This work revealed the solvent effect for positively participating in Li-ion primary solvation sheath and constructing SEI, providing a new possibility for developing LMA electrolytes," said Prof. CHEN.
This work was supported by Strategy Priority Research Program of CAS, R&D Projects in Key Areas of Guangdong Province, Science and Technology Innovation Foundation of Dalian, and Defense Industrial Technology Development Program. (Text by ZHU Shengdong)