Photocatalytic hydrogen evolution is a key technology for clean energy conversion, in which platinum (Pt) is widely used as an effective cocatalyst. The anchoring and dispersion of Pt play a decisive role in catalytic performance. However, achieving precise control over metal-support interactions at the atomic level remains challenging due to the chemical heterogeneity of catalyst surfaces.
In a recent study published in Angewandte Chemie International Edition, Prof. ZHOU Xukai and his colleagues from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) enhanced the photocatalytic hydrogen evolution performance of covalent organic frameworks (COFs) by introducing a conformational isomer strategy to precisely tune the nitrogen atom positions.
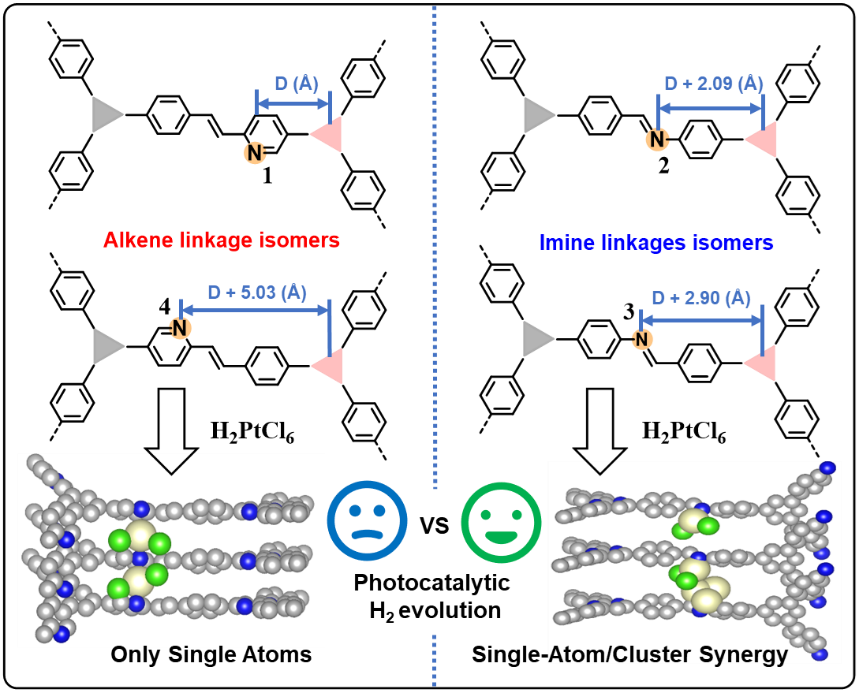
Constitutional isomerism in alkene/imine-linked COFs enables Ångström-scale positional control of N-anchoring sites (yellow) along hexagonal channel arms, dictating distinct Pt deposition configurations (single atoms/clusters) via in situ photodeposition of H2PtCl6 (Image by LI Hanxi and ZHOU Xukai)
COFs, with their programmable topology and well-defined pore environments, provide an ideal platform for studying metal-support interactions at the atomic level. In this work, researchers designed four COFs by combining trisubstituted aldehydes with trisubstituted aromatic amines or aromatic methyl compounds, yielding two olefin-linked COFs (COF-A1/A2) and two imine-linked COFs (COF-I1/I2). Although these materials share the same hexagonal pore topology, the positions of nitrogen anchoring sites were precisely tuned at the angstrom-level. After in situ photodeposition of Pt, the spatial arrangement of nitrogen atoms was found to play a decisive role in determining the dispersion and coordination environment of Pt species.
Multiple characterizations revealed that the imine-linked COF-I2 can simultaneously stabilize both Pt2+ single atoms and metallic Pt clusters, forming dual active sites. In contrast, the olefin-linked COF-A2 primarily anchors Pt single atoms. This structural difference directly leads to a remarkable disparity in photocatalytic performance: COF-I2-Pt exhibits a high hydrogen evolution rate of 26.72 mmol h-1 g-1, which is 6.1 times higher than that of its olefin-linked counterpart, COF-A2-Pt (4.40 mmol h-1 g-1). Under monochromatic light irradiation at 420 nm, COF-I2-Pt achieves an apparent quantum efficiency of 12.1%.
Further mechanistic investigations revealed that the superior performance of COF-I2-Pt stems from the synergistic effect between Pt clusters and single atoms. The Pt clusters act as hole-relay centers, while single atoms serve as electron-trapping sites. The charge redistribution between them effectively promotes the separation of photogenerated electron-hole pairs and optimizes the kinetics of proton adsorption and reduction. Femtosecond transient absorption spectroscopy further confirms a prolonged lifetime of the key charge-separated state in COF-I2-Pt, which is the primary reason for its efficient hydrogen evolution.
"Our study provides a new route for the rational design of atomically precise photocatalysts," said Prof. ZHOU. "And the concept of 'nitrogen-shift engineering' can be extended to the design of other porous framework materials, offering important guidance for developing efficient energy conversion materials."