Plastics are one of the most produced synthetic materials and the largest commodities, making our lives convenient and facilitating industrial processes.
The global production of plastics was reported to reach 9.2 billion tons in 2020, and it is still increasing every year. Among them, polyolefins, represented by polyethylene (PE) and polypropylene (PP), account for about 55% of the total plastics. However, only about 10% of plastic wastes were recycled due to their chemical inertness.
Recently, a research group led by Prof. PAN Xiulian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved the upcycling of polyolefin waste plastics and carbon dioxide (CO2) to aromatics with a high selectivity. This study was published in the journal of National Science Review.
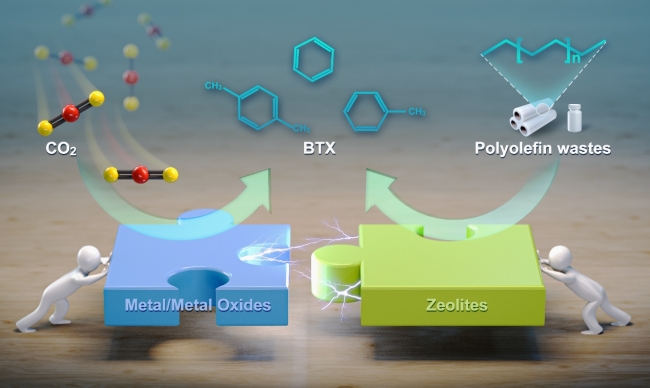
Scheme of upcycling polyolefin waste plastics and CO2 to aromatics (Image by DING Yi)
Inspired by the concept of oxide-zeolite (OXZEO) bifunctional catalysts for syngas conversion and CO2 hydrogenation, the researchers designed a bifunctional catalyst composed of ZSM-5 zeolite and Pt/MnOx for combined upcycling of polyolefin plastics and CO2 neutralization. ZSM-5 catalyzed the cracking of polyolefins and aromatization, generating hydrogen simultaneously while Pt/MnOx catalyzed the reaction of hydrogen with CO2, consequently driving the reaction toward aromatization. CO2 acted not only as a hydrogen scavenger but also as a carbon source that was incorporated into the aromatic-products.
With this bifunctional catalyst, the researchers proved that 1.0 kg polyolefin consumed 0.2 kg CO2, and 90% of the consumed CO2 was incorporated into the aromatic products. It yielded 0.63 kg aromatics, 0.28 kg liquefied petroleum gas (LPG) and naphtha, with monocyclic aromatics accounting for 87% and benzene, toluene, and xylene (BTX) accounting for 60% of the aromatics. By contrast, the conventional pyrolysis or hydrocracking processes produced only 0.33 kg aromatics at 300 °C and 1 MPa.
Furthermore, the researchers indicated that this designed bifunctional catalyst enabled upcycling of different polyolefins, including polyethylene, polypropylene, and their plastic products in different forms.
"We not only achieved the selective production of high value-added aromatic hydrocarbons such as BTX from waste polyolefin plastics under mild conditions but also provided a new strategy for the utilization of CO2," said Prof. PAN.