Although considerable efforts have been made in converting carbon dioxide to hydrocarbons via hydrogenation process, precise control of C-C coupling remains a challenge towards heavy olefins.
A research team led by Dr. SUN Jian and Prof. GE Qingjie in Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences reported a CO2 hydrogenation to olefin process that reaches 72% of alkenes selectivity and 50.3% of heavy alkenes (C4-C18), among which linear α-olefins accounts for 80%. Their findings were published online in Communications Chemistry.
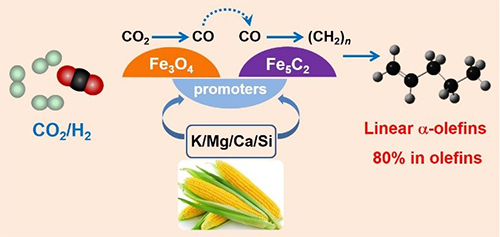
A schematic of CO2 hydrogenation to linear a-olefins catalyzed by iron catalysts with bio-promoters. (Image by SUN Jian)
The process is catalyzed by carbon supported iron, commonly used in C-C coupling reactions, with multiple alkali promoters extracted from waste biomass, corncob.
The design is based on the synergistic catalysis of mineral elements in biomass enzyme on which CO2 can be directly converted into carbohydrate. The mineral elements from corncob may promote the surface enrichment of potassium, suppressing the secondary hydrogenation of alkenes on active sites.
Furthermore, the carburization of iron species was obviously enhanced to form more Fe5C2 species, achieving well matching of reverse water-gas shift reaction and subsequent C-C coupling.
The utilization of biopromoters will be a starting point for the fabrication of efficient, green biological catalysts.
This study paves a new path for the synthesis of high value chemicals by utilizing CO2 and H2. And it provides an important approach for dealing with the intermittency of renewable sources (sun, wind and so on) by storing energy in chemicals. (Text by SUN Jian)