Chemical reactions are usually accompanied by thermal effects, inevitably resulting in temperature change in the reaction system. Therefore, temperature is an important parameter in reactions, which can affect chemical thermodynamics and reaction kinetics.
Precise measurement of the temperature near or at active sites inside a single catalyst particle during catalysis is important for establishing the reaction mechanism and developing the microscopic reaction kinetics.
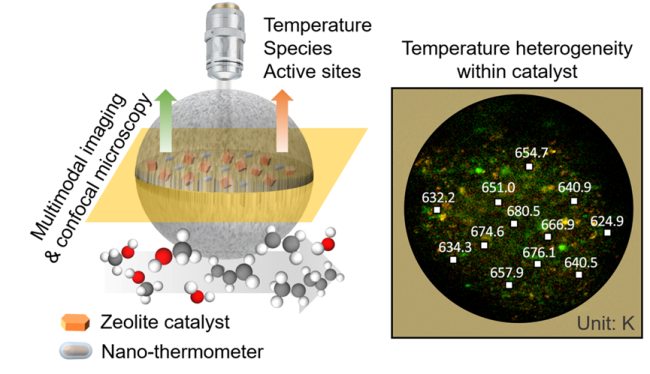
Recently, a research team led by Prof. YE Mao and Prof. LIU Zhongmin from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a three-dimensional spatiotemporal-resolved technique for the measurement of temperature distribution inside a single industrial zeolite-catalyst particle.
This study was published in the Journal of the American Chemical Society on Feb. 9.
Confocal up-conversion luminescence technique for the spatiotemporal measurements of temperature within individual catalyst particles (Image by GAO Mingbin)
The size of zeolite catalyst particles used in typical industrial processes is generally tens to hundreds of microns. However, the currently used thermocouples and infrared thermal imaging can only measure the surface temperature of the catalyst, and the spatial resolution is in millimeters.
To solve this problem, the researchers developed an imaging method with a spatial resolution of 800 nm, realizing the dynamic measurement of the three-dimensional spatiotemporal distribution of temperature inside the industrial zeolite catalyst particle during the methanol-to-olefins (MTO) reactions.
They developed this up-conversion confocal microscopic imaging technique by implanting the up-conversion nano-thermometer with high-temperature resistance into industrial zeolite catalyst particles using a microfluidic chip.
Furthermore, the researchers developed multimodal imaging techniques, i.e., confocal fluorescence and confocal infrared microscopy, and investigated the effects of zeolite contents and particle size on the spatiotemporal distribution of temperature inside the catalyst particles. They have revealed the utilization of active sites and the evolutions of reaction intermediates during MTO reactions affected by heterogeneous temperature distribution.
"This technique provides a new path to understand the heat transfer in catalyst particles toward rational design and optimization of industrial catalysts and catalysis," said Prof. YE.
This work was supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of CAS.