With the development of economy, the demand of basic olefins materials such as ethylene and propylene gradually increases in China. And due to the special Chinese energy structure which is lacking of petroleum and rich of coal relatively. It is necessary to produce more olefins products from coal instead of petroleum.
The research team led by Prof. LIU Zhongmin and Prof. WEI Yingxu in Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reveals the fundamental mechanism of Dalian methanol to olefins (DMTO) process. This new finding was published as “Hot Paper” in Angew. Chem. Int. Ed. entitled as “Direct mechanism of the first carbon-carbon bond formation in the methanol-to-hydrocarbons process.” (DOI: 10.1002/anie.201703902). And it has been selected as “Inside Back Cover” paper.
In order to parallel to the development of DMTO process, scientists in DICP have been always investigating the methanol conversion mechanism. It’s because the mechanism understanding can enhance the performance of catalyst, optimize the reaction conditions, and develop new catalysts with high selectivity.
Scientists have detected the initial formation of ethene product during the MTO reaction. And they have captured surface methoxy species (SMS) and trimethyloxonium (TMO) on the catalyst surface by solid-state nuclear magnetic resonance (ssNMR) spectroscopy.
More importantly, some surface methyleneoxy-analogue species, which were originated from activated DME, were observed directly by in situ ssNMR spectroscopy for the first time. They were recognized to be the most crucial intermediate for the formation of the first C-C bond. New insights into the formation of the first C-C bond are provided based on both experimental evidence and theoretical calculations. Therefore, the above results suggested SMS/TMO-mediated DME/methanol activation over an acid zeolite catalyst. And they proved a direct fundamental mechanism of DMTO process.
This new finding linked the direct mechanism of the initial methanol conversion and the indirect mechanism of the efficient methanol conversion. It has established a complete reaction course for methanol conversion over acid zeolite catalysts and enriched the fundamental theory of C1 catalytic chemistry.
Previously, scientists from DICP directly observed two important intermediates, including methylbenzenium cations and methylcyclopentenyl cations over silicoaluminophosphate (SAPO) zeolites during MTO reaction. This finding substantially proved the mechanism of “hydrocarbon pool” (HCP). Moreover, they also confirmed two catalytic cycles which include paring mechanism and side-chain methylation mechanism ( J. Am. Chem. Soc.2012, 134 (2), 836–839; Angew. Chem. Int. Ed.2013, 52(44), 11564-11568).
The DMTO process was a creative technology developed by DICP of CAS researchers in the last decade for the conversion of MTO. Coal is used as the main raw materials in this process. So far, DMTO process has achieved totally 23 licenses approved with a total ole?ns production capacity of 13.13 Mt/a. And 12 commercial DMTO units have been put into stream with ole?ns production capacity of 6.46 Mt/a.
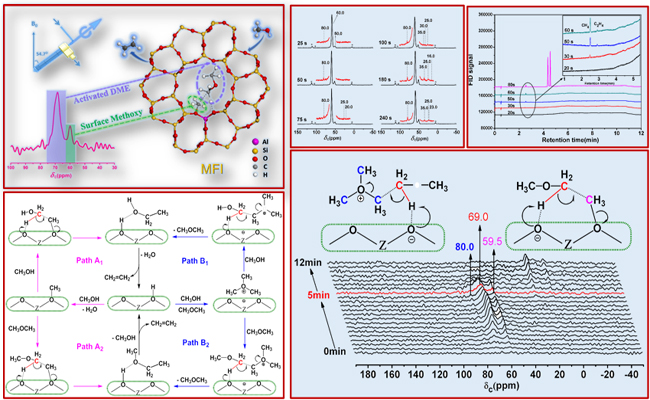
The mechanism for the first C-C bond formation in methanol to hydrocarbon reaction (Image by WU Xinqiang)
This work was supported by National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, iChEM and the Youth Innovation Promotion Association of the Chinese Academy of Sciences. (Text by XU Shutao and WU Xinqiang, Image by WU Xinqiang)
Dr. WANG Yongjin
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84374221
E-mail: wangyj@dicp.ac.cn