Classical strong metal-support interaction (SMSI) describes that reducible oxide migrates to the surface metal nanoparticles (NPs) to obtain metal@oxide encapsulation structure during high-temperature H2 thermal treatment, resulting in high selectivity and stability.
However, the encapsulation structure inhibited the adsorption and dissociation of reactant molecular over metal leading to low activity, especially for the hydrogenation reaction.
Recently, a research group led by Prof. LIU Yuefeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has proposed a new migration strategy, in which the TiO2 selectively migrated to second oxide support rather than the surface of metal NPs in Ru/(TiOx)MnO catalysts, boosting the CO2 reduction to CO via reverse water-gas shift reaction.
This study was published in Nature Catalysis on Oct. 9.
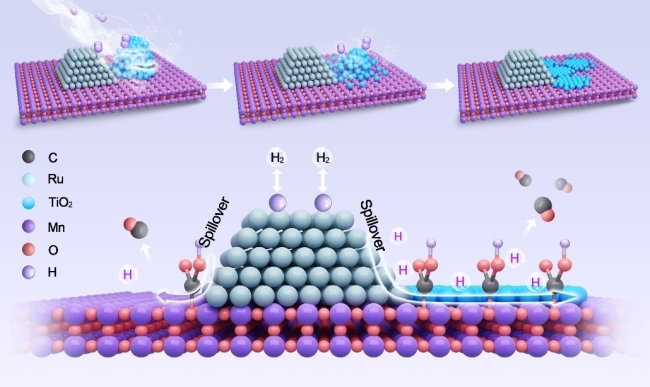
In-situ generation of highly efficient H-transport channel for CO2 reduction to CO(Image by Hui Kang)
The researchers achieved a controlled migration by utilizing the strong interaction between TiO2 and MnO in Ru/(TiOx)MnO catalysts during H2 thermal treatment, and TiO2 spontaneously re-dispersed on the MnO surface, avoiding the formation of TiOx shell on Ru NPs for the ternary catalyst (Ru/TiOx/MnO).
Meanwhile, high-density TiOx/MnO interfaces generated during the process, acting as a highly efficient H transportation channel with low barrier, and resulting in enhanced H-spillover for the migration of activated H species from metal Ru to support for consequent reaction.
The Ru/TiOx/MnO catalyst showed 3.3-fold catalytic activity for CO2 reduction to CO compared with Ru/MnO catalyst. In addition, the Ti/Mn support preparation was not sensitive to the crystalline structure and grain size of TiO2 NPs. Even the mechanical mixing of Ru/TiO2 and Ru/MnOx enhanced the activity.
Moreover, they verified that the synergistic effect of TiO2 and MnO didn't alter the catalytic intrinsic performance, and efficient H transport provided a large number of active sites (hydroxyl groups) for the reaction process.
"Our study provides references for the design of novel selective hydrogenation catalysts via the in-situ creation of oxide-oxide interfaces acting as hydrogen species transport channels," said Prof. LIU.
This work was supported by the NSFC of China, the Liaoning Revitalization Talents Program, the Dalian National Laboratory for Clean Energy and the AI S&T Program of Yulin Branch, the Dalian National Laboratory for Clean Energy of CAS.