It would be a sustainable way of replacing fossil fuels, reducing CO2 emissions, and facilitating the storage of renewable energy, if H2 used for converting CO2 was supplied by water electrolysis with renewable electricity. To precisely control the activation of CO2 as well as C-C coupling, has always been the most challenging problem in CO2 hydrogenation, which is also the key to the high-efficient utilization of CO2.
Recently, a research team led by Dr. SUN Jian and Prof. GE Qingjie at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences realized a one-step high-yield synthesis of isoparaffins from CO2 hydrogenation, catalyzed by a multifunctional Na–Fe3O4/HMCM-22 catalyst. Their findings were published in ACS Catalysis.
This team has focused on catalytic hydrogenation of CO2 to high-value fuels and chemicals, and has achieved the direct hydrogenation of CO2 to gasoline (Nature Communications), linear α-olefins (Communications Chemistry), and light olefins (Catalysis Science & Technology) by designing catalysts with multiple active sites.
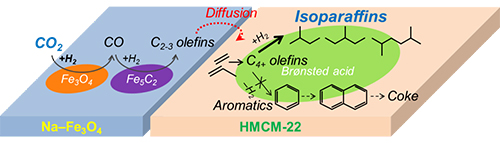
Reaction scheme of isoparaffin synthesis and coke formation during CO2 hydrogenation. (Image by WEI Jian)
In this study, researchers enabled a well matching of three tandem reactions comprising reverse water-gas shift, C-C coupling and isomerization through designing a multifunctional Na–Fe3O4/HMCM-22 catalyst. They achieved a selectivity of 82% among hydrocarbons for C4+ hydrocarbons, of which isoparaffins could account for 74%, while CO selectivity was as low as 17% at a CO2 conversion of 26%.
The unique pore structure and appropriate Br![]() nsted acid properties of HMCM-22, which effectively suppressed aromatization whilst promoting isomerization, are responsible for their high yields to isoparaffins.
nsted acid properties of HMCM-22, which effectively suppressed aromatization whilst promoting isomerization, are responsible for their high yields to isoparaffins.
In addition, researchers explored the reaction scheme of isoparaffin synthesis and coke formation during CO2 hydrogenation, and investigated the method for zeolite regeneration.
This study enriches the design of highly selective catalysts for CO2 hydrogenation into high-value chemicals.
This work was supported by the National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, and the Youth Innovation Promotion Association of Chinese Academy of Sciences. (Text by WEI Jian)