Supported oxides are widely used in many important catalytic reactions, in which the interaction between oxide catalyst and oxide support is critical but still remains elusive.
Recently, a research team led by Prof. BAO Xinhe and Prof. FU Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) disentangled local interfacial confinement and remote spillover effects in oxide-oxide interaction.
This work was published in the Journal of the American Chemical Society on July 26.
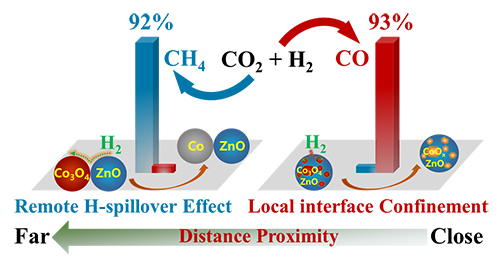
Schematic depiction of the effect of remote H spillover and local interfacial bonding on CO2 hydrogenation in oxide-oxides catalysts (Image by DONG Cui)
The researchers constructed a chemically bonded oxide-oxide interface by chemical deposition of Co3O4 onto ZnO powder (Co3O4/ZnO), in which complete reduction of Co3O4 to Co0 had been strongly impeded. They revealed that the local interfacial confinement effect between Co oxide and ZnO support helped to maintain a metastable CoOx state (mainly CoO) in CO2 hydrogenation reaction, producing 93% CO. In contrast, a physically contacted oxide-oxide interface was formed by mechanically mixing Co3O4 and ZnO powders (Co3O4-ZnO), in which reduction of Co3O4 to Co0 was promoted, demonstrating a quick increase of CO2 conversion to 45% and a high selectivity towards CH4 (92%) in the CO2 hydrogenation reaction. This interface effect was ascribed to unusual remote spillover of dissociated hydrogen species from ZnO nanoparticles to the neighboring Co oxide nanoparticles.
"Our study illustrates the equally important but opposite local and remote effects at the oxide-oxide interfaces, and the distinct oxide-oxide interactions contribute to many diverse interface phenomena in oxide-oxide catalytic systems," said Prof. FU.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Photon Science Center for Carbon Neutrality.