Dynamic control of oxide nanostructures is crucial for the design of advanced oxide catalysts, which is also significant for understanding the active site and reaction mechanism in oxide catalysis.
Recently, a research team led by Prof. FU Qiang and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) discovered that the reversible dynamic conversion between Cr oxide (CrOx) nanoislands with the same thickness and CrOx clusters with the identical size supported on Au(111) surface under different redox treatments.
This work was published in Proceedings of the National Academy of Sciences of the United States of America on May 23.
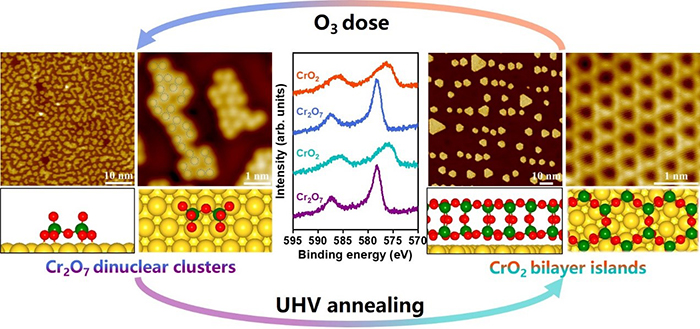
Dynamic transformation between bilayer islands and dinuclear clusters of Cr oxide on Au(111) through environment and interface effects (Image by NING Yanxiao and YI Zhiyu)
The researchers grew two kinds of well-defined CrOx nanostructures on Au(111) and unambiguously identified them by scanning tunneling microscopy and theoretical calculations.
They found that the CrOx nanoislands featured a CrO2-bilayer (BL) structure consisting of two Cr2O3 monolayers bridged by one layer of O, and the CrOx clusters had a Cr2O7 stoichiometry. Moreover, oxidation treatment in O3 could disperse the CrO2-BL nanoislands into the Cr2O7-dinuclear clusters, which could be dynamically converted back to the CrO2-BL by annealing in an ultrahigh vacuum.
Furthermore, surface science experiments and theoretical simulations revealed that both surface oxygen atoms dissociated from O3 and the confinement effect of the Au substrate played important roles in the formation of the Cr2O7-dinuclear clusters.
"The oxide nanocatalysts with controlled size and structure can be stabilized by the specific environment and oxide-metal interface", Prof. FU said.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Liaoning Revitalization Talents Program. (Text/image by NING Yanxiao and YI Zhiyu)