A research group led by Assoc. Prof. LUO Nengchao and Prof. WANG Feng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences realized selective control of photocatalytic coupling reaction of alcohols.
This study was published in Journal of the American Chemical Society on Oct. 10.
Radicals are common intermediates in photocatalytic conversions, with open-shell electronic structures. They are highly active and readily adsorb on the surface of semiconductors strongly, resulting in a variety of reactions. Solution can influence the product selectivity and quantum yield of the photocatalytic reaction.
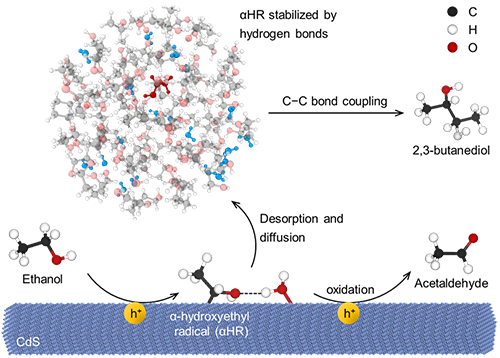
Steering the reaction paths of αHRs toward C-C bond coupling by forming hydrogen bonds (Image by MU Junju and GAO Zhuyan)
In this work, the researchers found that adding 5 vol% water to ethanol solution could increase the selectivity of 2,3-butanediol generated by photocatalytic coupling of ethanol from 37% to 57%, with a reaction rate 2.4 times of the original.
Using radical trapping experiments, deuterium experiments, DFT calculations, and molecular dynamics simulations, they found that the introduction of a small amount of water enabled the intermediate α-hydroxyethyl radical (αHR) to form hydrogen bonds with solvent molecules both on the catalyst surface and in bulk solution. With the help of hydrogen bonding, αHR tended to desorb from the catalyst surface and was stabilized in solution, avoiding the predominant oxidation of αHR to acetaldehyde and reverse reaction between αHRs and H· that recoverd ethanol.
"Our study revealed that non-chemical bonding interactions can steer the reaction paths of radicals for selective photocatalysis," said LUO.
This work was supported by the Ministry of Science and Technology of the People's Republic of China, the National Natural Science Foundation of China, the Liaoning Revitalization Talents Program, the Fundamental Research Funds for the Central Universities, the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy, and by Dalian Institute of Chemical Physics, CAS. (Text by GAO Zhuyan)