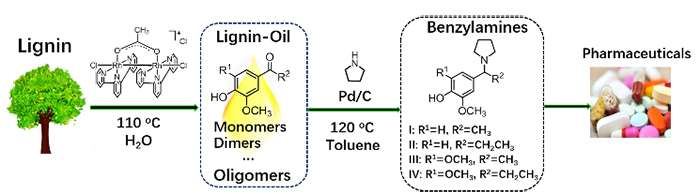
Benzylamines, one type of N-functionalized aromatics, are widely used as precursors of pharmaceuticals and synthetic dyes. Catalytic conversion of lignin into value-added N-containing chemicals such as benzylamines is significant to bring the biorefinery concept into reality.
Recently, Prof. LI Changzhi and Prof. ZHANG Tao's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences designed a new strategy for direct transformation of lignin β-O-4 model compounds, the most abundant segment in lignin, into benzylamines.
This study was published in Angew. Chem. Int. Ed. on July 23.
Sustainable production of benzylamines from lignin (Image by LI Changzhi)
This proposed strategy involved dehydrogenation of Cα-OH, hydrogenolysis of the Cβ-O bond and reductive amination in the presence of Pd/C catalyst. Experimental data suggested that the dehydrogenation reaction proceeded over the other two reactions and secondary amines served as both reducing agents and amine sources in the transformation.
Based on the above findings, the researchers demonstrated the feasibility for the production of bio-based amines from lignin in a two-step process. First, the lignin-oil was obtained through mild depolymerization of lignin catalyzed by a binuclear rhodium complex. Then, direct conversion of lignin-oil with pyrrolidine occurred over Pd/C, producing major four benzylamines.
This work represents a first example of synthesis of benzylamines from lignin and provides a new opportunity for the sustainable synthesis of heteroatom functionalized chemicals from renewable biomass.
This study was supported by National Key R&D Program of China, the National Natural Science Foundation of China, and the Royal Society International Collaboration Award. (Text by LI Changzhi)