Lignin is the aromatic polymer fragment of lignocellulose, which is the richest renewable aromatic carbon source in nature. The depolymerization of lignin and the preparation of a wide variety of aromatic compounds can be achieved through lignin cleavage.
However, most current depolymerized strategies utilize species such as active hydrogen (H) and oxygen (O) to obtain value-added aromatics from lignin. Since the lignin itself contains only carbon (C), H and O, the product composition from lignin is limited to C, H and O.
The future bio-refinery demands a broad spectrum of fine chemicals, especially those containing elements other than C, H and O.
Heteroatom-containing compounds have emerged as powerful reagents to participate in the novel bond cleavage in lignin; meanwhile, the obtained heteroatom-containing aromatics extend the application of lignin-derived products.
A group led by Prof. WANG Feng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reviewed the recent advances in the lignin C–C and C–O bond cleavages induced by heteroatoms X (N, Si, I and Li), which could generate functionalized products containing C–X and O–X bonds.
The review article was published in Chemical Society Reviews.
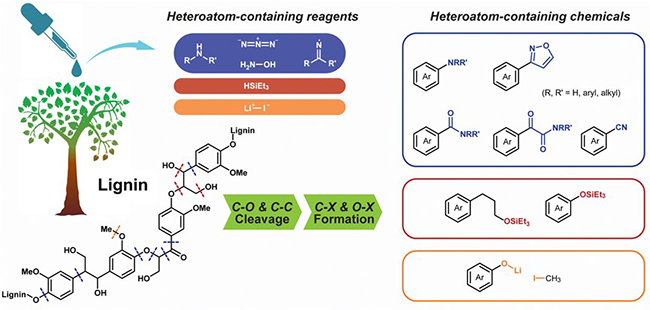
Heteroatom-containing reagents triggered the catalytic cleavage of lignin linkages and functionalization of products simultaneously. (Image by LI Hongji)
Catalytic depolymerization methods have been investigated using various nitrogen reagents (including amines, hydroxylamine, imine and azide), silanes and lithium iodide. These heteroatom X (N, Si, S and Li)-containing reagents triggered the cleavage of lignin C–C and C–O bonds and contributed to formation of C–X and O–X bonds in products.
The functionalization with heteroatoms onto lignin derived aromatics provides valuable properties for further upgrading of lignin monomers in many applications such as in the polymer, agriculture, pharmaceutical, material and chemical industries.(Text by LI Hongji)