Classical molecular sieve membranes, with 3D microparticles and 2D nanosheets as primary building blocks, are promising in chemical separation.
Separation within such membranes relies on molecular movement and transport though their intrinsic or artificial nanopores. Since the weak connections by nature between the neighboring "bricks" usually result in intercrystalline gaps in membranes, the prevailing selectivity for classical molecular sieve membranes is moderate.
Recently, a research group led by Prof. YANG Weishen and Dr. BAN Yujie from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) proposed zero-dimensional molecular sieve membranes that could enhance the separation selectivity of hydrogen (H2) and carbon dioxide (CO2).
This study was published in Angewandte Chemie International Edition on July 16.
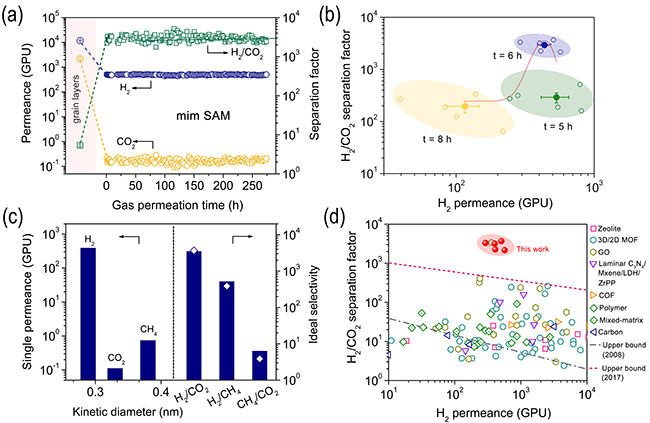
Gas permeation of SAM (Image by BAN Yujie)
"Zero-dimensional molecules, as primary building blocks in the proposed membrane, have the potential to absolutely eliminate intercrystalline gaps in membranes," said Dr. BAN.
The researchers fabricated the zero-dimensional molecular sieve membrane by orderly assembling zero-dimensional 2-methylimidazole (mim) molecules into unprecedented supramolecule array membranes (SAMs) through solvent-free vapor processing on a metal-organic framework.
In SAMs, the "zero-dimensional building blocks" together with supramolecule interactions resulted in the absence of the intercrystalline gaps, which guaranteed an effective mass-transfer through intermolecular spacings instead of an undesirable leakage through non-selective gaps.
In contrast to the classical transport though nanopores of membranes, selective transport through the intermolecular spacing of mim (~0.30 nm) was realized within SAMs, yielding an extremely precise sieving of H2 from CO2. The H2/CO2 selectivity was one order of magnitude higher than selectivities of the state-of-the-art classical membranes.
"Our study opens the door to create a variety of SAMs to distinguish the subtle size/shape differences of a pair of gas molecules," said Prof. YANG. "In the future, we will tailor the intermolecular spacing, control the assembly process, and enable a wide range of application of SAMs to energy-efficient chemical separation processes."
The above work was supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of CAS. (Text by BAN Yujie)