C-H bond is very important in organic chemistry. Chemical reactions related to the break and further synthesis of C-H bond require high activation energy and poor selectivity. Therefore, it's important to understand reaction mechanism of C-H bond.
Recently, a research team led by Prof. YANG Xueming and Prof. MA Zhibo from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. PAN Minghu from the Huazhong University of Science and Technology, discovered the C-H bond breaking reaction catalyzed by low-temperature photocatalysis on the surface of titanium oxide, and explained the reaction mechanism at the single molecular level.
The results were published in The Journal of Physical Chemistry Letters on November 10.
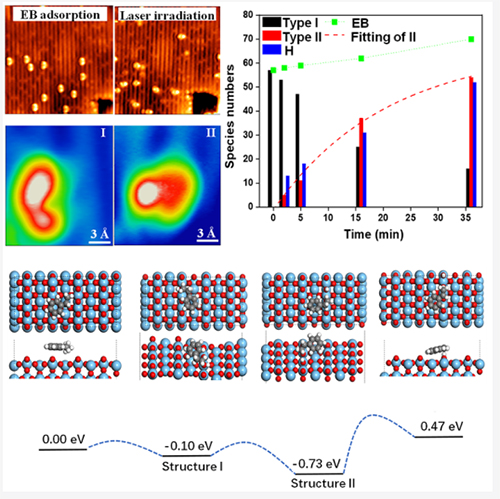
High-resolution STM visualizing the intermediate states of the reaction and the reaction pathway of EB dehydrogenation on TiO2 (Image by WANG Haochen)
The scientists used the rutile 110 surface of titanium oxide as the model system and the C-H bond molecule ethylbenzene as the model molecule.
They found that ethylbenzene could be induced to remove hydrogen at low temperature (77K) only by photoinduced reaction. They also experimentally observed the images of the reaction steps by in-situ tracking single molecules.
Prof. PAN's team provided theoretical support for the theoretical interpretation of the image and confirmed the reaction process.
This work was supported by the Chinese Academy of Sciences Strategic Pioneer Science and Technology Special Project (Category B), the Chinese Academy of Sciences' Key Frontier Science Research Program, the National Key Research and Development Program of the Ministry of Science and Technology, and the National Natural Science Foundation of China. (Text by WANG Haochen)