Research groups led by Prof. ZHANG Donghui and Associate Prof. LIU Shu from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently revealed Feshbach Resonances in the F+H2O→HF+OH reaction. The results were published in Nature Communications.
Feshbach resonances of a chemical reaction are transiently trapped quantum states along the reaction coordinate in the transition-state region. They not only have a profound influence on both the rate and product distribution, but also provide sensitive probes to the potential energy surface (PES) of the reaction.
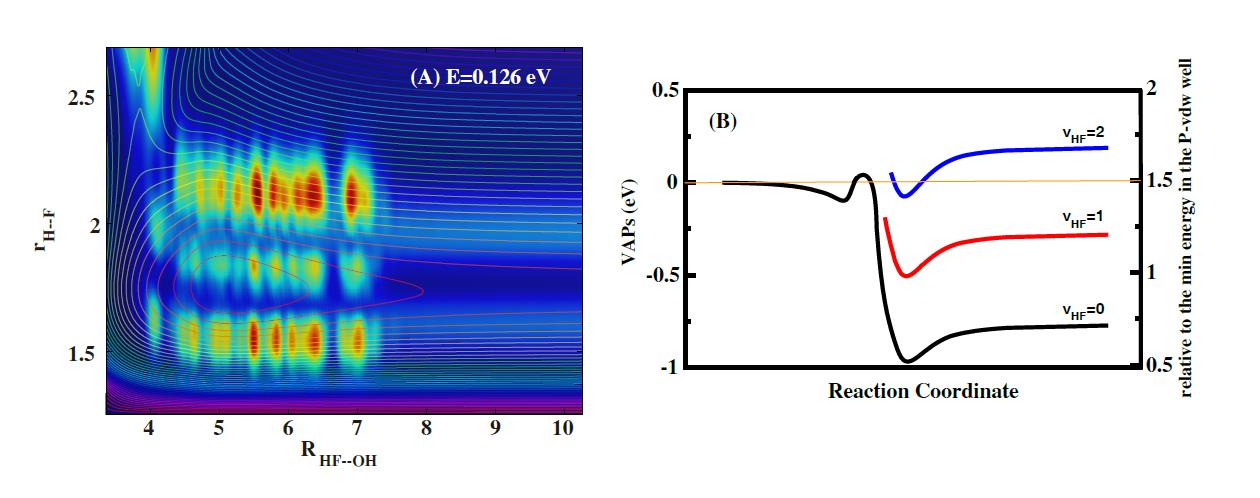
(A)Scattering wave functions;(B)vibrational adiabatic potentials analyses. (Image by LIU Shu)
Over the past decades, great efforts have been devoted to detecting resonances in chemical reactions and to studying their structures and dynamics. Through close interactions between theory and experiment, our understanding of Feshbach resonances in triatomic reaction such as the F/Cl+H2 (HD) reactions is now highly sophisticated. One of the major challenges for reaction dynamics is to achieve such sophisticated understanding of systems involving more than three atoms,as the first step is the systems involving four atoms.
As a result, the F+H2O reaction has emerged as the benchmark system for such a purpose. Similar to the F+H2 system, the F+H2O→HF+OH reaction is highly exothermic and has a low and “early” barrier on the ground electronic state.
There is a relatively deep vanr der Waals (vdw) well in the entrance channel, and a hydrogen bond well in the exit channel. In an earlier photodetchment study of FH2O?, Feshbach resonances have been discovered trapped in the HF-OH interaction well. However, it is not clear if these resonances are accessible by the F+H2O reaction. And how these resonances affect effect the reaction?
Recently, the ZHANG’s group performed an accurate state-to-state quantum scattering study of the title reaction by using the time-dependent wave packet method on an accurate newly constructed PES. The total reaction probabilities exhibit many pronounced oscillatory structures especially for collision energy below 0.2 eV.
Analysis of scattering wave functions and vibrational adiabatic potentials (VAP) reveal that these oscillating structures originate from the Feshbach resonance states trapped in the HF(v’=2)-OH VAP well, producing mainly HF(v’=1) product. However, different from the F+H2 reaction in which the HF bond softening creates a peculiar well on the H-HF(v’=3) VAP, the VAP well for the F+H2O reaction is mainly hydrogen bond between HF and OH in nature.
This work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and DICP. (Text and Image by LIU Shu)