Reactions occurring at a carbon atom with a tetrahedral environment proceeding through the back-side attack Walden inversion mechanism are one of the most important and useful classes of reactions in chemistry. The bimolecular nucleophilic substitution (SN2) reaction is the most common reaction of that type. In a SN2 reaction, a nucleophile approaches a saturated carbon from one side, displaces a leaving group on the opposite side of the carbon atom, resulting in inversion of the carbon center. Decades of extensive studies revealed that a gas-phase SN2 reaction with a center barrier exhibits an inverse secondary kinetic isotope effect (KIE) on thermal rate constant at room temperature, i.e. kH/kD < 1 when isotopically substituted atom not directly involved in the reaction. In contrast, on the cross sections it shows a large normal secondary isotope effect with reaction threshold energy substantially higher than calculated barrier height. However, the origin of this intriguing difference on the isotope effects on the kinetic and cross sections as well as the high energy threshold remain unclear.
A research term led by prof. ZHANG Donghui from State Key Laboratory of Molecular Reaction Dynamics, Dalian Institute of Chemical Physics, Chinese Academy of Sciences has carried out an accurate quantum dynamics study of the H' + CH4 → CH3H' + H substitution reaction and its isotope analogies. The reaction is the simplest reaction proceeding through the back-side attack Walden inversion mechanism, with a D3h transition state and a static barrier height of 1.6 eV. It is very similar to the gas-phase SN2 reactions with central barriers, except that there exist pre- and post-reaction wells in SN2 reactions stemming from strong ion-dipole interaction between reagents/products. They found that the reaction threshold energy is considerably higher than the barrier height, and the reaction manifests different isotope effect. In term of cross section beyond reaction threshold energy, it has a large normal secondary isotope effect with the reactivity for the H' + CH4 → CH3H' + H reaction substantially larger than that for H' + CHD3 → CD3H' + H reaction. However, in term of thermal rate constant it has a reverse secondary KIE with the rate for H + CH4 slightly smaller than that for H + CHD3.
In order to explore the dynamics origin of these different isotope effects, they analyzed the change of the umbrella angle of the non-reacting methyl group as the reaction proceeds. According to the minimum energy path (MEP), the umbrella angle should change synchronously during the reaction with the incoming of H atom and elongation of the breaking CH bond to reach 90° at the static saddle point. However, it turned out that for the H' + CHD3 substitution reaction the umbrella motion of the non-reacting CD3 group is slow in responding to the attack of the incoming H atom during the reaction. The reaction does not proceed along MEP as shown by the black reaction path in the figure. Instead, it proceeds on the red path with the umbrella angle larger than 90° at the dynamical saddle point and the corresponding barrier height higher than the static barrier height. Because the umbrella motion depends on the masses of the methyl group, isotope replacement of D atom to H atom can enhance the reactivity substantially, resulting in a large normal isotope effect on cross sections. Furthermore, because the initial umbrella excitation of methyl group can accelerate the umbrella motion during reaction substantially, it can dramatically enhance the reactivity. As a result, the thermal rate constant for the reaction is exclusively determined by the contributions from the umbrella excited states. As the contributions from the umbrella excited states for the H + CD3H reaction beat those for the H + CH4 reaction, the reaction exhibits an inverse secondary KIE.
All the phenomena found from this study for the H+CH4 substitution reactions, including higher threshold energy, a reverse secondary KIE at room temperature, and a large normal isotope effect for reaction cross section, have been observed for gas-phase SN2 reactions with barriers. We anticipate that the mechanisms uncovered from this study play important roles in these gas-phase SN2 reactions. Therefore, this study only not represents the first accurate theoretical study of a Walden inversion reaction which provided unprecedented dynamical details and a clear physical picture of the dynamics, but also sheds light on the dynamics of gas-phase SN2 reactions.
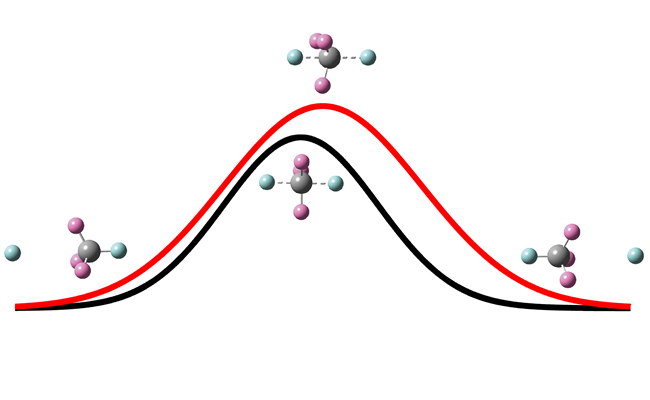
H' + CH4 → CH3H' + H substitution reaction (Image by ZHANG Donghui)
The related work was published in Nature Communications entitled as “Dynamical barrier and isotope effects in the simplest substitution reaction via Walden inversion mechanism”. This study was financially supported by the Natural Science Foundation of China, Ministry of Science and Technology of China, and the Chinese Academy of Sciences. (Text and Image by ZHANG Donghui) (CN 约稿)
Contact:
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn