Recently, Dr. WANG Lianyue and Prof. GAO Shuang at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences reported aerobic oxidative cleavage of 1,2-diols and insights into the reaction pathway. Their findings were published in Communications Chemistry.
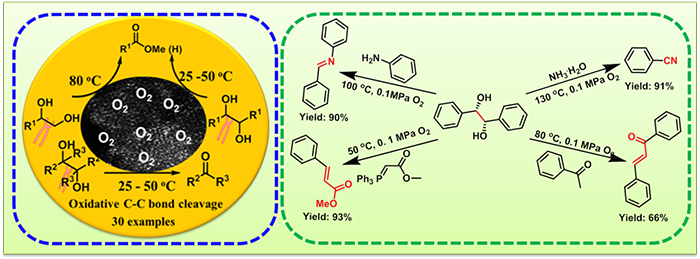
Aerobic oxidative cleavage of 1,2-diols catalyzed by atomic-scale cobalt and its application in tandem reactions (Image by WANG Lianyue)
The oxidative cleavage of the C-C bonds in 1,2-diols to the corresponding carbonyl compounds is one of the most important transformation in synthetic organic chemistry. The classical methods for cleaving the C-C bond of 1,2-diols use stoichiometric or excess amount oxidants, such as periodic acid and its salts or lead tetraacetate.
Up to now, these methods remain the most widely used for vicinal diols cleavage. But these oxidants produce equimolar quantities of toxic waste and cannot meet the current environmental sustainable development needs. Therefore, the development of catalytic oxidation processes with a clean oxidant such as molecular oxygen is an alternative and promising protocol.
Although the early homogeneous transition-metal catalytic systems are capable of cleaving simple diols with molecular oxygen, most of them are still suffering from drawbacks such as low product selectivity, limited substrate range, the use of expensive noble metals (e.g., Pd, Ru), the required harsh conditions, and no reusability of the catalyst.
Therefore, development of a sustainable and cost efficient heterogeneous catalysts to achieve oxidative cleavage of 1,2-diols to synthesize carbonyl compounds under mild and green conditions has become an important consideration for the design of catalysts.
Scientists reported a Co-based heterogeneous catalyst supported on nitrogen-doped carbon materials with improved catalytic performance in the aerobic oxidative cleavage of a variety of 1,2-diols into esters, aldehydes or ketones under mild conditions with O2 or air as the oxidant. For example, the oxidative cleavage of pinacol was achieved at ambient temperature and air pressure. It was also effective for the application of oxidative cleavage of 1,2-diols in tandem reactions.
Based on a large number of control experiments, kinetics and isotope labeling experiments, the oxidative cleavage processes of terminal diols and pinacol were studied respectively. Mechanistic insights into the cleavage of terminal diols revealed that a sequence reaction, involving stepwise oxidation/nucleophilic addition /C-C bond cleavage was disclosed. Mechanistic investigations on pinacol give a conclusion that trans isomer, meso-hydrobenzoin was fast cleaved faster than the cis isomer, (S,S)-hydrobenzoin and that the reaction followed a type II mechanism via the formation of a monodentate complex between diol and active oxidant species, which then oxidatively collapses to the carbonyl product in a two-electron oxidation.
Moreover, mild reaction conditions, non-noble metal catalyst and excellent reusability of the catalyst made the present catalytic system more competitive than the existing ones.
This study will provide a new idea for the development of catalysts for oxidative cleavage of C-C bonds.
This work was supported by the National Natural Science Foundation of China, Major Science and Technology Innovation Project. (Text by WANG Lianyue)