Research News
-
 06 25, 2021Researchers Fabricate Bio-friendly X-ray Detectors based on Metal-free Perovskite Single CrystalsScientists fabricated halide-modulated self-assembly of metal-free perovskite single crystals for bio-friendly X-ray detection.
06 25, 2021Researchers Fabricate Bio-friendly X-ray Detectors based on Metal-free Perovskite Single CrystalsScientists fabricated halide-modulated self-assembly of metal-free perovskite single crystals for bio-friendly X-ray detection.
Metal-free halide perovskites are novel candidates for ferroelectrics and X-ray detection. However, the molecular self-assembly of these perovskites and its influence remain unexplored.
Recently, a research group led by Prof. LIU Shengzhong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) fabricated halide-modulated self-assembly of metal-free perovskite single crystals for bio-friendly X-ray detection.
This study was published in Matter on June 14.
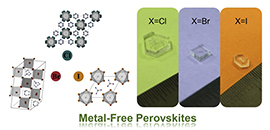
Metal-free perovskite series, DABCO-NH4X3 (X = Cl, Br, I), exhibit a remarkable variety of perovskite-type structures through halide modulation, therefore their single crystals grown at the same condition show different crystal morphology (Image by DUAN Lianjie)
Molecular self-assembly plays a critical role in crystal engineering for the design and fabrication of novel metal-free perovskite compounds.
After preparing DABCO-NH4X3 (X = Cl, Br, I) single crystals, the researcher investigated halide-modulated molecular assembly via hydrogen bonding in metal-free perovskites and its influence on their crystal packing, band nature, mechanical and electrical properties, as well as final optoelectronic performance.
They found that the crystal DABCO-NH4I3 with superior in-plane charge transport and lower charge effective mass exhibited higher carrier mobility. Therefore, it presented better X-ray detection sensitivity, reaching 567 μC Gyair-1 cm–2. Meanwhile, they demonstrated the feasibility of X-ray imaging had a well-defined 'heart' image.
A variety of non-metallic and organic groups readily were available for the A, B and X in crystal DABCO-NH4I3, resulting in fine-tuned properties and free of associated toxicity.
"This work benefited the understanding of molecular self-assembly behavior and was intended to inspire activities to study an assortment of novel ABX3 perovskite materials for potential biological and therapeutic applications," said Prof. LIU.
This work was supported by the National Key Research and Development Program of China, the Key Program project of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the 111 Project. (Text by DUAN Lianjie) -
 06 24, 2021Surface Oxygenate Species Enhance Cobalt-catalyzed Fischer-Tropsch SynthesisScientists observed the migration of TiOx species to metallic cobalt surface during the reduction process employing the in situ environmental scanning transmission electron microscopy (ESTEM).
06 24, 2021Surface Oxygenate Species Enhance Cobalt-catalyzed Fischer-Tropsch SynthesisScientists observed the migration of TiOx species to metallic cobalt surface during the reduction process employing the in situ environmental scanning transmission electron microscopy (ESTEM).
Carbide supported metal catalysts are promising due to the special properties of metal carbide and the interactions between metals and the carbide supports. However, surface oxide species are inevitable in carbide materials, and their roles in metal-carbide interaction still remain unclear.
Recently, Assoc. Prof. LIU Yuefeng's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LIU Xi from Shanghai Jiao Tong University, observed the migration of TiOx species to metallic cobalt surface during the reduction process employing the in situ environmental scanning transmission electron microscopy (ESTEM).
This work was published in ACS Catalysis on June 19.
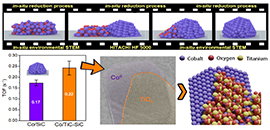
The reduction process for cobalt species and the origin for the improved intrinsic activity (Image by JIANG Qian)
Co/TiC-SiC catalysts presented higher Fischer-Tropsch Synthesis (FTS) activity than Co/SiC owing to the stronger metal support interaction that resulted in the better dispersion of Co NPs as well as the improved turnover frequency (TOF).
The researchers achieved direct structural observation at nanoscopic scale for the metal-support interaction and revealed that the migration of surface TiOx species on the metallic active site significantly involved in the modulation of the interaction between metal and TiC substrate.
The formation of Co-TiOx-TiC heterojunction structure over Co/TiC-SiC catalyst not only benefited the dispersion of Co NPs, but also rendered the superior intrinsic FTS activity.
"This work paves the way for the future rational design of advanced catalysts through moderate metal-support interaction by employing surface oxide species on carbide materials," said Prof. LIU.
This work was supported by the National Natural Science Foundation of China, Liaoning Revitalization Talents Program, and China Postdoctoral Science Foundation. (Text by JIANG Qian) -
 06 24, 2021Study Reveals Formation Mechanism of First Carbon-carbon Bond in Methanol-to-Olefins Process
06 24, 2021Study Reveals Formation Mechanism of First Carbon-carbon Bond in Methanol-to-Olefins Process
A joint research team led by Prof. LIU Zhongmin, Prof. WEI Yingxu, and Prof. XU Shutao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed the mechanism underlying the formation of the first carbon-carbon (C-C) bond formation during the methanol-to-olefins (MTO) process.
This study was published in Chem on June 23.
Prof. ZHENG Anmin's group from Innovation Academy for Precision Measurement Science and Technology of CAS was also involved in the study.
The first C-C bond in the MTO process is formed at the initial stage of the reaction. There is no direct method to elucidate the bond formation/reaction mechanism due to the difficulty in capturing intermediate species.
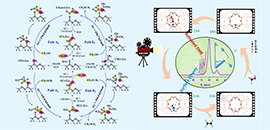
Revealing the whole first C-C bond formation processes in MTO reaction: based on the in situ NMR spectroscopic evidences and advanced ab initio molecular dynamics (AIMD) theoretical calculation method (Image by XU Shutao and WEI Yingxu)
"We investigated the direct C-C bond formation mechanism during the initial MTO reaction over HSSZ-13 zeolite with an 8-membered ring and a chabazite topological structure," said Prof. XU.
They detected the evolution of the organic species on the SSZ-13 catalyst surface during the methanol conversion. For the first time, they directly captured the surface ethoxy species (SES), the critical species containing the initial C-C bond under real MTO reaction conditions at the initial reaction stage.
Moreover, the researchers employed the ab initio molecular dynamics (AIMD) theoretical calculation simulation to predict and present the visualized and complete process of initial C-C bond formation starting from the C1 reactants and C1 intermediates.
Based on the experimental and theoretical evidence, they established the complete and feasible initial C-C bond formation routes, namely, surface methoxy species (SMS)/trimethoxyonium (TMO) mediated methanol/dimethyl ether (DME) activation with a synergistic effect from SMS and the negatively charged framework oxygen atoms to form SES.
"Our study not only sheds light on the controversial issue of the first C-C bond formation in the MTO process, but also enriches the fundamental theory of C1 catalytic chemistry," said Prof. WEI.
This work was supported by the National Natural Science Foundation of China, Strategic Priority Research Program of the Chinese Academy of Sciences, iChEM, the Youth Innovation Promotion Association of the Chinese Academy of Sciences and Liaoning Revitalization Talents Program. (Text by XU Shutao and WEI Yingxu) -
 06 11, 2021Study Reveals How Y-box Binding Protein 1 Promotes Cancer MetastasisScientists revealed that the nucleotide binding protein Y-box binding protein 1 can promote the metastasis of liver cancer by regulating the biosynthesis of microRNA.
06 11, 2021Study Reveals How Y-box Binding Protein 1 Promotes Cancer MetastasisScientists revealed that the nucleotide binding protein Y-box binding protein 1 can promote the metastasis of liver cancer by regulating the biosynthesis of microRNA.
Metastasis of cancer is the main cause of cancer relevant death as well as the main challenge of cancer treatment.
Recently, a group led by Prof. PIAO Hailong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed that the nucleotide binding protein Y-box binding protein 1 (YB1) can promote the metastasis of liver cancer by regulating the biosynthesis of microRNA (miRNA).
This study was published in Cancer Communications on June 10.
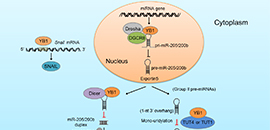
YB1 triggers cell invasion and cancermetastasis by regulating the miRNA200/205b-ZEB axis (Image by LIU Xiumei and CHEN Di)
The researchers found that YB1 could significantly inhibit the biosynthesis and expression of miRNA-200b and miRNA-205 by interacting with miRNA microprocessor complex DgCR8, Dicer and terminal UTP transferase TUTs. Then, it up-regulated the expression of zinc-finger E-box binding homeobox 1 (ZEB1), which is a key protein for cancer metastasis.
The development of lung cancer metastasis was simulated by a mouse model injected through tail vein, and the researchers found that YB1 could promote lung metastasis of hepatocellular carcinoma cells.
Furthermore, transcriptomics data of clinical tissues showed that the expression of YB1 was positively correlated with ZEB1 in liver cancer tissues, and negatively correlated with the expression of miRNA200 and miRNA205b. The miRNA200/205b-ZEB1 signaling pathway was significantly correlated with the prognosis of patients.
This work was supported by National Natural Science Foundation of China, Innovation program of science and research from the DICP, and the construction of Liaoning Provincial Cancer Center. (Text by LIU Xiumei and CHEN Di) -
 06 10, 2021Newly Developed Ion-conducting Membrane Improves Performance of Alkaline-zinc Iron Flow BatteryScientists from DICP developed layered double hydroxide (LDH) membrane with high hydroxide conductivity and ion selectivity for alkaline-zinc iron flow battery.
06 10, 2021Newly Developed Ion-conducting Membrane Improves Performance of Alkaline-zinc Iron Flow BatteryScientists from DICP developed layered double hydroxide (LDH) membrane with high hydroxide conductivity and ion selectivity for alkaline-zinc iron flow battery.
Alkaline zinc-iron flow battery (AZIFB) is well suitable for stationary energy storage applications due to its advantages of high open-cell voltage, low cost, and environmental friendliness. However, it surfers from zinc dendrite/accumulation and relatively low operation current density.
Recently, a research group led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed layered double hydroxide (LDH) membrane with high hydroxide conductivity and ion selectivity for alkaline-zinc iron flow battery.
The study was published in Nature Communications on June 7.
Selective ions transport and the hydroxide ions transport in LDHs (Image by HU Jing)
In order to enhance the operating current density of AZIFB, the researchers added LDHs nano materials into the AZIFB and designed a LDHs-based composite membrane with high performance. High selectivity and superb hydroxide ion conductivity were achieved through the combination of the well-defined interlayer gallery with a strong hydrogen bond network along 2D surfaces.
They identified that surface -OH groups of LDHs layer could assist the conduction of OH- by promoting proton transfer away from one water molecule to the original OH-.
Because of the high ionic conductivity, the LDHs-based membrane enabled the AZIFB to operate at 200 mA cm-2, along with an energy efficiency of 82.36%.
"This study offers a new insight to design and manufacture high-performance membranes for AZIFB," said Prof. LI. (Text by YUAN Zhizhang) -
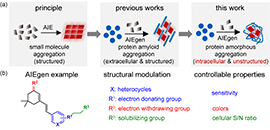 06 08, 2021Researchers Design Sensors to Detect Amorphous Protein AggregationResearchers from DICP rationally designed amorphous protein aggregation sensors from aggregation induced emission probes (AIEgens).
06 08, 2021Researchers Design Sensors to Detect Amorphous Protein AggregationResearchers from DICP rationally designed amorphous protein aggregation sensors from aggregation induced emission probes (AIEgens).
Most current protein aggregation sensors focus on amyloid proteins for their well-defined β-sheet stacked structure. Intracellular amorphous protein aggregation with random and indefinite structure is usually envisioned to be untargetable or undruggable by small molecule probes.
Recently, Prof. LIU Yu's group and Prof. PIAO Hailong's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. LIU Xiaojing from Shandong University, designed amorphous protein aggregation sensors from aggregation induced emission probes (AIEgens), and systematically investigated the structure-fluorescence relationship to dissect structural moieties.
This study was published in Angewandte Chemie International Edition on May 14.
Rational design of crystallization induced emission probes to detect amorphous protein aggregation in live cells (Image by SHEN Di)
This sensor achieved the selective detection via its inherent binding affinity towards amorphous aggregated proteins. Due to the low-polarity and high-viscosity microenvironment inside aggregated proteins, the environment-sensitive sensor turned on its fluorescence upon binding these aggregated proteins.
"This work may provide a new strategy for further design of imaging sensors, proteomics probes, and therapeutic reagents for amorphous aggregated protein," said Prof. LIU.
This work was supported by the National Natural Science Foundation of China, Liaoning Revitalization Talents Program, China Postdoctoral Science Foundation Grant, and Dalian Innovation Fund. (Text by SHEN Di)