Research News
-
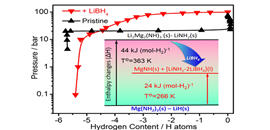 03 14, 2017Researchers Prepare Near Ambient Condition Hydrogen Storage MaterialDalian Institute of Chemical Physics (DICP) group led by Prof. CHEN Ping demonstrates that the synergy among LiBH4, Mg(NH2)2 and LiH, three of the most-investigated HLEs, can lead to a fully reversible hydrogenation and dehydrogenation cycle at temperatures below 373 K. The measured dehydrogenation enthalpy of this intriguing material is only 24 kJ(mol-H2)-1, which theoretically allows to de-hydrogenate the material at 273 K and 3.2 bar H2. Furthermore, experimentally it was shown that full H2 absorption can be achieved at 326 K and de-hydrogenation at 371 K, the lowest operating temperatures for hydrides of light elements as reported before.
03 14, 2017Researchers Prepare Near Ambient Condition Hydrogen Storage MaterialDalian Institute of Chemical Physics (DICP) group led by Prof. CHEN Ping demonstrates that the synergy among LiBH4, Mg(NH2)2 and LiH, three of the most-investigated HLEs, can lead to a fully reversible hydrogenation and dehydrogenation cycle at temperatures below 373 K. The measured dehydrogenation enthalpy of this intriguing material is only 24 kJ(mol-H2)-1, which theoretically allows to de-hydrogenate the material at 273 K and 3.2 bar H2. Furthermore, experimentally it was shown that full H2 absorption can be achieved at 326 K and de-hydrogenation at 371 K, the lowest operating temperatures for hydrides of light elements as reported before.
To find a suitable material to store hydrogen under moderate temperature and hydrogen pressure is a key roadblock towards the commercialization of hydrogen powered fuel cell vehicles. In the recent years, many hydrides of light elements (HLEs) have attracted considerable attention for use as on-board hydrogen storage materials due to their high volumetric and gravimetric hydrogen densities.
However, their application is limited by severe hydrogenation/de-hydrogenation kinetic barriers and poor intrinsic thermodynamics. Although several approaches have been reported to improve the material thermodynamic and kinetic properties, a material, which can fully satisfy the requirements such as fully reversible and operating near ambient temperature set by the automotive industry, was not found yet.
Dalian Institute of Chemical Physics (DICP) group led by Prof. CHEN Ping demonstrates that the synergy among LiBH4, Mg(NH2)2 and LiH, three of the most-investigated HLEs, can lead to a fully reversible hydrogenation and dehydrogenation cycle at temperatures below 373 K. The measured dehydrogenation enthalpy of this intriguing material is only 24 kJ(mol-H2)-1, which theoretically allows to de-hydrogenate the material at 273 K and 3.2 bar H2. Furthermore, experimentally it was shown that full H2 absorption can be achieved at 326 K and de-hydrogenation at 371 K, the lowest operating temperatures for hydrides of light elements as reported before.
The Pressure-Composition-Temperature desorption curves of +LiBH4 and Pristine (Image by WANG Han)
The DICP researchers attribute the exceptional material properties to a possible “solvent” like behavior of LiBH4 in stabilizing the intermediates and products of the hydrogenation/de-hydrogenation cycle. Such an understanding of the role of LiBH4 can devise design and development of multi-component HLEs systems in which one or multiple components may serve as a medium to modulate the thermodynamic and kinetic properties of hydrogenation and dehydrogenation.
This work was published on Advanced Energy Materials (DOI: 10.1002/aenm.201602456). This work is financially supported National Science Funds for Distinguished Young Scholars, the Collaborative Innovation Center of Chemistry for Energy Materials (2011-iChEM), CAS-Helmholtz JRG Project and National Natural Science Foundation of China. (Text and Image by WANG Han)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn -
 03 14, 2017DICP Researchers Reported Diverse Reactions of 2-Tosylmethylphenol with Allenic EsterThe Molecular Modeling and Design group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277;J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
03 14, 2017DICP Researchers Reported Diverse Reactions of 2-Tosylmethylphenol with Allenic EsterThe Molecular Modeling and Design group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277;J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
Ortho-Quinone methides (o-QMs) are important intermediates in organic synthesis and materials chemistry as well as biological processes. However, the asymmetric reactions with o-QMs generated in situ are unexplored.
The Molecular Modeling and Design Group from Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences has developed a highly efficient protocol for the preparation of optically active benzylic sulfones and 4-substituted chromans. This work has been published recently as a communication in Angew. Chem. Int. Ed. (DOI:10.1002/anie.201700250)
The Molecular Modeling and Design Group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277; J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
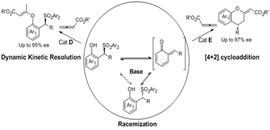
Different reaction pathways of 2-sulfonylalkyl phenols with allenic esters via o-QM intermediates: DKR versus [4+2] cycloaddition. (Image by LIU Yan and CHEN Ping)
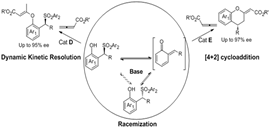
In this work, it was found the strong base Et3N can precede the racemization of the o-QMs precursor. So that it can afford the possibility for the design of dynamic kinetic resolution (DKR) involving the high reactive intermediates. This group reported the first DKR involving o-QMs intermediates. In the presence of Et3N and cinchonine-derived nucleophilic catalyst, the DKR of 2-sulfonylalkyl phenols with allenic esters afforded chiral benzylic sulfones with good to excellent enantioselectivities (85-95% ee). Furthermore, using 2-(Tosylmethyl) sesamols or 2-(Tosylmethyl) naphthols which can generate stable o-QMs as the substrates, a formal [4+2] cycloaddition was achieved, delivering 4-aryl- or alkyl-substituted chromans with excellent enantioselectivities (88-97% ee). This work also provided a new strategy to design new asymmetric reactions involving o-QMs.
The first author of this work is PhD candidate CHEN Ping, co-supervised by Prof. LIU Yan and Prof. LI Can. The research work was financially supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by LIU Yan and CHEN Ping)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn -
 03 14, 2017DICP Researchers Reported Diverse Reactions of 2-Tosylmethylphenol with Allenic EsterThe Molecular Modeling and Design group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277;J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
03 14, 2017DICP Researchers Reported Diverse Reactions of 2-Tosylmethylphenol with Allenic EsterThe Molecular Modeling and Design group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277;J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
Ortho-Quinone methides (o-QMs) are important intermediates in organic synthesis and materials chemistry as well as biological processes. However, the asymmetric reactions with o-QMs generated in situ are unexplored.
The Molecular Modeling and Design Group from Dalian Institute of Chemical Physics (DICP), Chinese Academy of Sciences has developed a highly efficient protocol for the preparation of optically active benzylic sulfones and 4-substituted chromans. This work has been published recently as a communication in Angew. Chem. Int. Ed. (DOI:10.1002/anie.201700250)
The Molecular Modeling and Design Group has been working on developing new heterogeneous methodolgies for a long time (Angew. Chem. Int. Ed., 2002, 21; Angew. Chem. Int. Ed., 2007, 6861), especially on the water-oil biphases system which has been widely used in a series of asymmetric catalytic reactions (Chem. Eur. J., 2004, 2277; J. Catal., 2007, 360; Green Chem., 2011, 1983; Angew. Chem. Int. Ed., 2012, 13159). In the previous work, this group realized the first conjugate addition of trityl thiol to in situ generated o-QMs. It was catalyzed by a chiral organic base with excellent enantioselectivities by using the advantage of water-oil biphases catalytic system. It consist of spatial separation between the organic-base catalyst, reactants in the organic phase, and the inorganic base in the aqueous phase, thus to suppress the racemic background reaction.
Different reaction pathways of 2-sulfonylalkyl phenols with allenic esters via o-QM intermediates: DKR versus [4+2] cycloaddition. (Image by LIU Yan and CHEN Ping)
In this work, it was found the strong base Et3N can precede the racemization of the o-QMs precursor. So that it can afford the possibility for the design of dynamic kinetic resolution (DKR) involving the high reactive intermediates. This group reported the first DKR involving o-QMs intermediates. In the presence of Et3N and cinchonine-derived nucleophilic catalyst, the DKR of 2-sulfonylalkyl phenols with allenic esters afforded chiral benzylic sulfones with good to excellent enantioselectivities (85-95% ee). Furthermore, using 2-(Tosylmethyl) sesamols or 2-(Tosylmethyl) naphthols which can generate stable o-QMs as the substrates, a formal [4+2] cycloaddition was achieved, delivering 4-aryl- or alkyl-substituted chromans with excellent enantioselectivities (88-97% ee). This work also provided a new strategy to design new asymmetric reactions involving o-QMs.
The first author of this work is PhD candidate CHEN Ping, co-supervised by Prof. LIU Yan and Prof. LI Can. The research work was financially supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by LIU Yan and CHEN Ping)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn -
 03 07, 2017Researchers Report an Efficient Catalyst System to Produce Primary AminesA team led by prof. ZHANG Tao and WANG Aiqin, Dalian Institute of Chemical Physics, has developed an efficient approach to produce primary amines in good to excellent yields from biomass-derived aldehydes and ketones, by using a bi-functional and robust Ru/ZrO2 catalyst in aqueous ammonia under quite mild conditions. More interesting, they also demonstrated the feasibility of producing ethanolamine directly from lignocellulose, with an overall yield of 10%.
03 07, 2017Researchers Report an Efficient Catalyst System to Produce Primary AminesA team led by prof. ZHANG Tao and WANG Aiqin, Dalian Institute of Chemical Physics, has developed an efficient approach to produce primary amines in good to excellent yields from biomass-derived aldehydes and ketones, by using a bi-functional and robust Ru/ZrO2 catalyst in aqueous ammonia under quite mild conditions. More interesting, they also demonstrated the feasibility of producing ethanolamine directly from lignocellulose, with an overall yield of 10%.
Utilization of lignocellulosic biomass for production of liquid fuels and chemicals is being considered as one of the promising strategies to reduce CO2 emissions. As lignocellulose is composed of C, H and O, most of the products resulting from lignocellulose are oxygenates or hydrocarbons. In comparison, transforming lignocellulose into more valuable N-containing compounds has rarely been achieved owing to the lack of efficient amination methods for biomass-derived molecules.
A team led by prof. ZHANG Tao and WANG Aiqin, Dalian Institute of Chemical Physics, has developed an efficient approach to produce primary amines in good to excellent yields from biomass-derived aldehydes and ketones, by using a bi-functional and robust Ru/ZrO2 catalyst in aqueous ammonia under quite mild conditions. More interesting, they also demonstrated the feasibility of producing ethanolamine directly from lignocellulose, with an overall yield of 10%.
Production of primary amines from biomass-derived aldehydes and ketones (Image by LIANG Guanfeng and WANG Aiqin)
Primary amines are value-added intermediates in fine chemical industry. In particular, ethanolamine has a large market of approximately 2 MT/a as a widely used absorbent for CO2 sequestration in power plants. This work can thus provide a sustainable avenue to primary amines from renewable biomass.
This work was published on Angew. Chem. Int. Ed..The research work was financially supported by the National Natural Science Foundation of China, the National Key Projects for Fundamental Research and Development of China, and the Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by LIANG Guanfeng and WANG Aiqin)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn -
 03 07, 2017Researchers Report an Efficient Catalyst System to Produce Primary AminesA team led by prof. ZHANG Tao and WANG Aiqin, Dalian Institute of Chemical Physics, has developed an efficient approach to produce primary amines in good to excellent yields from biomass-derived aldehydes and ketones, by using a bi-functional and robust Ru/ZrO2 catalyst in aqueous ammonia under quite mild conditions. More interesting, they also demonstrated the feasibility of producing ethanolamine directly from lignocellulose, with an overall yield of 10%.
03 07, 2017Researchers Report an Efficient Catalyst System to Produce Primary AminesA team led by prof. ZHANG Tao and WANG Aiqin, Dalian Institute of Chemical Physics, has developed an efficient approach to produce primary amines in good to excellent yields from biomass-derived aldehydes and ketones, by using a bi-functional and robust Ru/ZrO2 catalyst in aqueous ammonia under quite mild conditions. More interesting, they also demonstrated the feasibility of producing ethanolamine directly from lignocellulose, with an overall yield of 10%.
Utilization of lignocellulosic biomass for production of liquid fuels and chemicals is being considered as one of the promising strategies to reduce CO2 emissions. As lignocellulose is composed of C, H and O, most of the products resulting from lignocellulose are oxygenates or hydrocarbons. In comparison, transforming lignocellulose into more valuable N-containing compounds has rarely been achieved owing to the lack of efficient amination methods for biomass-derived molecules.
A team led by prof. ZHANG Tao and WANG Aiqin, Dalian Institute of Chemical Physics, has developed an efficient approach to produce primary amines in good to excellent yields from biomass-derived aldehydes and ketones, by using a bi-functional and robust Ru/ZrO2 catalyst in aqueous ammonia under quite mild conditions. More interesting, they also demonstrated the feasibility of producing ethanolamine directly from lignocellulose, with an overall yield of 10%.
Production of primary amines from biomass-derived aldehydes and ketones (Image by LIANG Guanfeng and WANG Aiqin)
Primary amines are value-added intermediates in fine chemical industry. In particular, ethanolamine has a large market of approximately 2 MT/a as a widely used absorbent for CO2 sequestration in power plants. This work can thus provide a sustainable avenue to primary amines from renewable biomass.
This work was published on Angew. Chem. Int. Ed..The research work was financially supported by the National Natural Science Foundation of China, the National Key Projects for Fundamental Research and Development of China, and the Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by LIANG Guanfeng and WANG Aiqin)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn -
 03 07, 2017Researchers in DICP Prepare a Gold Catalyst for Chemoselective Hydrogenation of Functionalized NitroarenesProf. ZHANG Tao’s group in Dalian Institute of Chemical Physics has synthesized a gold catalyst for chemoselective hydrogenation of functionalized nitroarenes. By using the Zinc-Aluminum hydrotalcite (ZnAl-HT) supported thiolated Au25 nanoclusters (NCs) as the precursor of the catalyst, they prepared the high selectivity Au catalyst for the selective hydrogenation of functionalized nitroarenes in wide temperature and time ranges.
03 07, 2017Researchers in DICP Prepare a Gold Catalyst for Chemoselective Hydrogenation of Functionalized NitroarenesProf. ZHANG Tao’s group in Dalian Institute of Chemical Physics has synthesized a gold catalyst for chemoselective hydrogenation of functionalized nitroarenes. By using the Zinc-Aluminum hydrotalcite (ZnAl-HT) supported thiolated Au25 nanoclusters (NCs) as the precursor of the catalyst, they prepared the high selectivity Au catalyst for the selective hydrogenation of functionalized nitroarenes in wide temperature and time ranges.
Functionalized aromatic amines are important industrial intermediates for pharmaceuticals, agrochemicals, fine chemicals, dyes and polymers. They were generally synthesized by chemoselective hydrogenation of the corresponding functionalized nitroarenes. However, the traditional noble-metal catalysts were active not only for the reduction of the nitro group but also for the hydrogenation of other unsaturated functional groups existing in the same molecule.
Prof. ZHANG Tao's group in Dalian Institute of Chemical Physics has synthesized a gold catalyst for chemoselective hydrogenation of functionalized nitroarenes. By using the Zinc-Aluminum hydrotalcite (ZnAl-HT) supported thiolated Au25 nanoclusters (NCs) as the precursor of the catalyst, they prepared the high selectivity Au catalyst for the selective hydrogenation of functionalized nitroarenes in wide temperature and time ranges.
Previously, Prof. ZHANG's group have developed a rationally designed FeOx supported Pt single-atom and pseudo single-atom catalyst for the hydrogenation of functionalized nitro groups. By precisely controlling the reaction time and temperature, they obtained the good activity and selectivity for this kind of reaction. But the nitro group and the other unsaturated groups were competitively adsorbed on the catalyst so that excessive hydrogenation was inevitable while the concentration of the nitro group decreased.
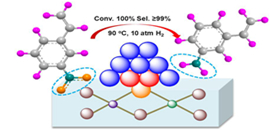
Figure 1. The reaction model of selective hydrogenation of 3-nitrostyrene over the Au25/ZnAl-HT-300 catalyst.(Image by TAN Yuan and LIU Xiaoyan)
Au catalyst exhibits high selectivity for various oxidation and hydrogenation reactions, due to the weak adsorption of reactant and product molecular. It has becoming one of the most important research projects in Prof. ZHANG's group in the last decade. They have done a series of important work on this topic. (Nano Today, 2013, 8, 403-416; J. Catal., 2013, 308, 258-271, 50th Anniversary Special Issue). This time they prepared a gold catalyst with high selectivity for the hydrogenation of functionalized nitroarenes to the target product.
Compared with traditional gold catalysts, the thiolated Au25 NCs supported on the ZnAl-hydrotalcite was highly resistant to sintering, with well controlled size (around 2.0 nm) even after calcination at 500 oC. The formation of the Au-S-Zn bond and the epitaxial interaction between gold and the support might contribute to the stabilization of the gold nanoclusters. Besides, the results of the control experiments suggested the catalyst was inert to adsorb other unsaturated groups, thus made it as a potential candidate for selective hydrogenation reactions. It is quite different from the behavior of the supported platinium-group metals.
Figure 2. The evolution of the product distribution of the chemoselective hydrogenation reaction of 3-nitrostyrene over the Au25/ZnAl-HT-300 catalyst.(Image by TAN Yuan and LIU Xiaoyan)
This work was published on Angew. Chem. Int. Ed..The research work was financially supported by the National Natural Science Foundation of China, the National Key Projects for Fundamental Research and Development of China, the Youth Innovation Promotion Association CAS, and the Strategic Priority Research Program of the Chinese Academy of Sciences. The work was also supported by the BL 14W at the Shanghai Synchrotron Radiation Facility (SSRF) for the XAFS experiments.(Text and Image by TAN Yuan and LIU Xiaoyan)
Dr. LU Xinyi
Dalian Institute of Chemical Physics, Chinese Academy of Sciences,
457 Zhongshan Road, Dalian, 116023, China,
Tel: 86-411-84379201
E-mail: luxinyi@dicp.ac.cn