In a study published in Journal of the American Chemical Society, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) revealed that only three water molecules can separate Ba and OH in neutral BaOH(H2O)n (n = 1−5) clusters, and uncovered the microscopic mechanism of alkali dissolution exemplified by hydrated BaOH clusters.
The dissolution of alkali species in water plays a crucial role in areas such as energy storage and pharmaceuticals. It is unclear how water molecules initiate the dissociation of alkali due to the difficulty in probing hydrogen bonding, proton transfer, and electrostatic interactions in complex solvent environments. Neutral hydrated alkali clusters serve as ideal models for investigating these early solvation processes.
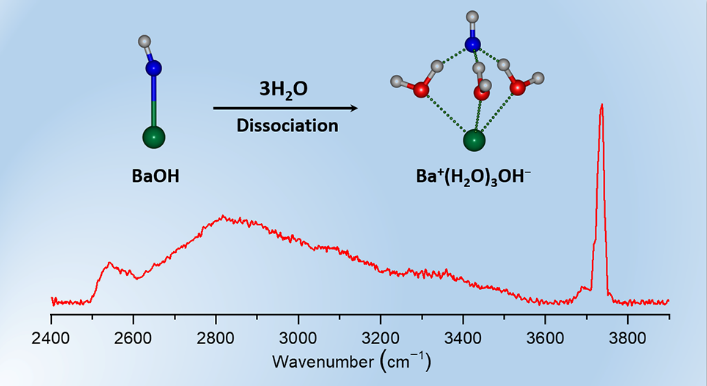
Experimental IR-VUV spectra and identified structures of hydrated BaOH clusters (Image by YAN Wenhui)
In this study, researchers developed a neutral cluster infrared spectroscopy station based on infrared excitation and vacuum ultraviolet threshold photoionization (IR-VUV), which enables high-sensitivity IR spectral detection, structural characterization, and reactivity studies of mass-selected neutral clusters.
Utilizing this station and tabletop extreme ultraviolet sources, researchers measured the IR spectra of neutral BaOH(H2O)n (n = 1−5) clusters. Besides, they compared the experimental spectra with high-level quantum chemical harmonic calculations and anharmonic molecular dynamics simulations.
Researchers found that when n = 1 and 2, water molecules interact directly with BaOH via O–H⋯O hydrogen bonds without dissociation of Ba and OH; When n ≥ 3, Ba and OH dissociate to form a solvent-shared ion pair structure. Electronic structure analysis showed that as the number of water molecules increased, charge transfer reduced electrostatic attraction, and the formation of a hydrogen-bond network promoted the separation of Ba and OH.
This work proposes a model for understanding electrostatic and inductive interactions between ionic species and water molecules. It provides new insights into the early solvation in closed-shell systems, and paves the way for further size-resolved studies of solvation mechanisms in chemical and biological processes.