Hydrogen spillover is a well-established concept in heterogeneous catalysis where hydrogen atoms migrate across solid surfaces and interfaces. However, whether a similar process could occur for metal species has been an open question.
In a study published in Nature Communications, a research team led by Prof. FU Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) demonstrated a new form of metal spillover that is mediated by water adlayers, and revealed that metal species can spontaneously migrate across solid interfaces under mild and humid conditions.
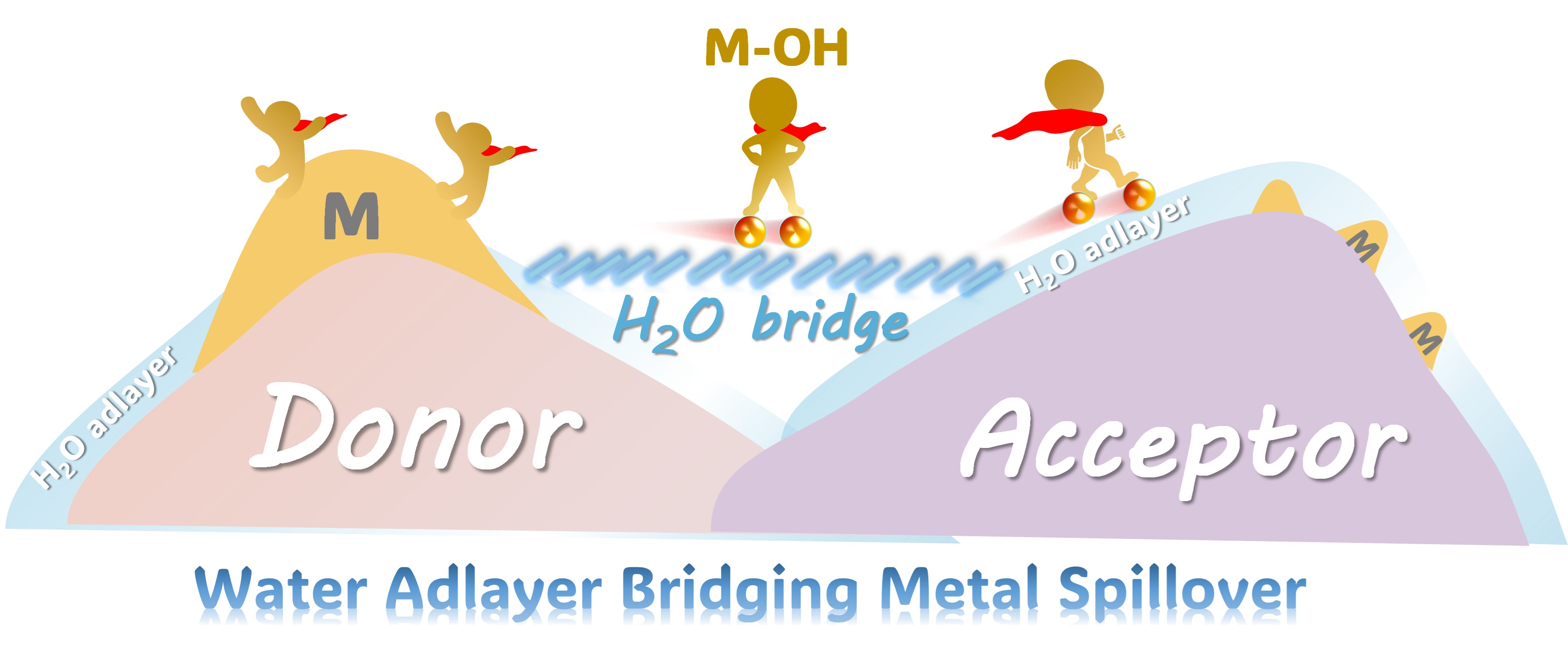
Schematic illustration of water-adlayer-mediated metal spillover under mild conditions (Image by FAN Yamei)
Researchers found that when hydrophilic supports such as oxides, carbides, or sulfides are exposed to a humid environment, a thin layer of water is formed on the surfaces. This water adlayer acts as a molecular bridge, enabling metal species, exemplified by copper, to migrate between different supports. The metal migration occurs through hydroxylated intermediates (M–OH) and proceeds spontaneously at room temperature without requiring high-temperature activation commonly used in catalyst preparation.
Then, researchers showed that this spillover phenomenon is not limited to copper, and other metals including ruthenium, cobalt, and nickel also exhibit similar migration behavior. By tuning the surface hydrophilicity, the density of hydroxyl group, and the degree of interfacial contact, they were able to control metal mobility and distribution.
Catalysts synthesized through this spillover route exhibited improved low-temperature activity in reactions, including carbon monoxide oxidation, reverse water–gas shift, ammonia selective catalytic reduction, and hydrogen cyanide oxidation. These catalysts outperformed those prepared using conventional impregnation methods.
Mechanistic analysis showed that water adlayers and dissociated hydroxyl groups together form a hydration lubrication layer that lowers the diffusion barrier for metal atom migration. This mechanism is conceptually analogous to hydrogen spillover but represents the first direct extension of spillover to metal species. "Our study highlights how interfacial water layers and surface hydroxyls dynamically regulate catalyst structures and activities even under ambient conditions," said Prof. FU.
This work not only provides a fundamental understanding of water-mediated metal migration, but also offers a new strategy for designing dynamic catalysts operable at low temperatures, promoting the development of energy-efficient catalytic systems.