Organic redox-active molecules (ORAMs) are abundant and diverse, offering significant potential for cost-effective and sustainable energy storage, particularly in aqueous organic flow batteries (AOFBs). However, ensuring the stability of the ORAMs during the charge and discharge process is critical, as side reactions can deactivate them and eliminate their redox activity. Air stability remains a challenge for many ORAMs, complicating their practical use.
Recently, a research group led by Prof. LI Xianfeng and Prof. ZHANG Changkun from the Dalian Institute of Chemical Physics (DlCP) of the Chinese Academy of Sciences (CAS) developed novel naphthalene derivatives with active hydroxyls and dimethylamine scaffolds that were stable in air and served as effective catholytes for AOFBs. This study, published in Nature Sustainability, demonstrates that these novel ORAMs can achieve long-term stable cycling even under air-atmosphere conditions.
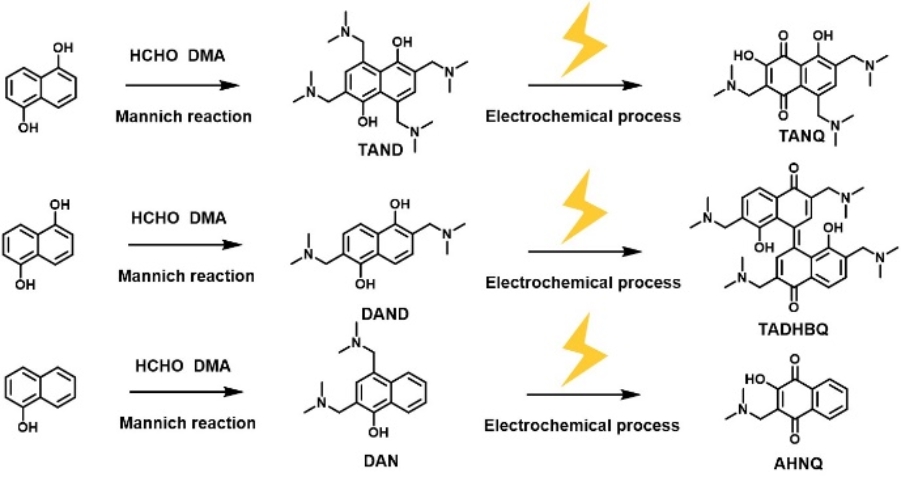
Chemical and electrochemical reaction of the naphthalene derivatives (Image by ZHAO Ziming and ZHANG Changkun)
ORAMs are challenged with instability and high cost, particularly when used without inert gas protection. This can lead to irreversible capacity loss and a reduced battery lifespan.
In this study, the researchers synthesized active naphthalene derivatives using a scalable approach that combined chemical and in situ electrochemical methods. This approach simplified the purification process and significantly reduced the cost of molecular synthesis.
Moreover, the researchers demonstrated specific structure changes in the naphthalene derivatives during the electrochemical process. The as-prepared naphthalene derivatives featured a multisubstituted framework with hydrophilic alkylamine scaffolds, which not only protected against potential side reactions but also improved their solubility in aqueous electrolytes.
The 1.5 mol/L naphthalene-based AOFB displayed stable cycling performance for 850 cycles (about 40 days) with a capacity of 50 Ah/L. Remarkably, even with continuous air flow in the catholyte, the naphthalene-based AOFB could run smoothly for approximately 600 cycles (about 22 days) without capacity and efficiency decay. This demonstrated that the naphthalene-based catholyte had excellent air stability.

Apilot-scale naphthalene-based flow stack (Image by ZHAO Ziming and ZHANG Changkun)
Furthermore, the researchers scaled up the preparation of naphthalene derivatives to the kilogram scale (5 kg per pot). Pilot-scale battery stacks containing these naphthalene derivatives achieved an average system capacity of approximately 330 Ah. They exhibited remarkable cycling stability over 270 cycles (about 27 days), with a capacity retention of 99.95% per cycle.
"This study is expected to open a new field in the design of air-stable molecular for sustainable and air-stable electrochemical energy storage," said Prof. LI.