Glycerol is a key byproduct of biodiesel fabrication, and its production has increased proportionally with the growth of biodiesel production.
Glycerol electrooxidation is considered as an innovative strategy due to its low theoretical potential and the elimination of the need for external toxic oxidative agents, pressurized oxygen, and high temperature. Ni-based catalysts featured with abundant supply, low cost, and corrosion resistance were used for glycerol electrooxidation reactions. However, the onset potential was subjected to the high generation potential of NiOOH, which is 1.35 V vs. RHE, leading to inferior electrooxidation activity.
Recently, a joint research team led by Prof. WU Zhong-Shuai and Prof. XIAO Jianping from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences has developed a highly active Cu-doped NiCo alloy (Cu-NiCo/NF) catalyst, and constructed an energy-saving nitrate reduction system coupled with glycerol oxidation. This approach has achieved high glycerol electrooxidation activity and selectivity to produce formate at room temperature. This study was published in Angewandte Chemie-International Edition.
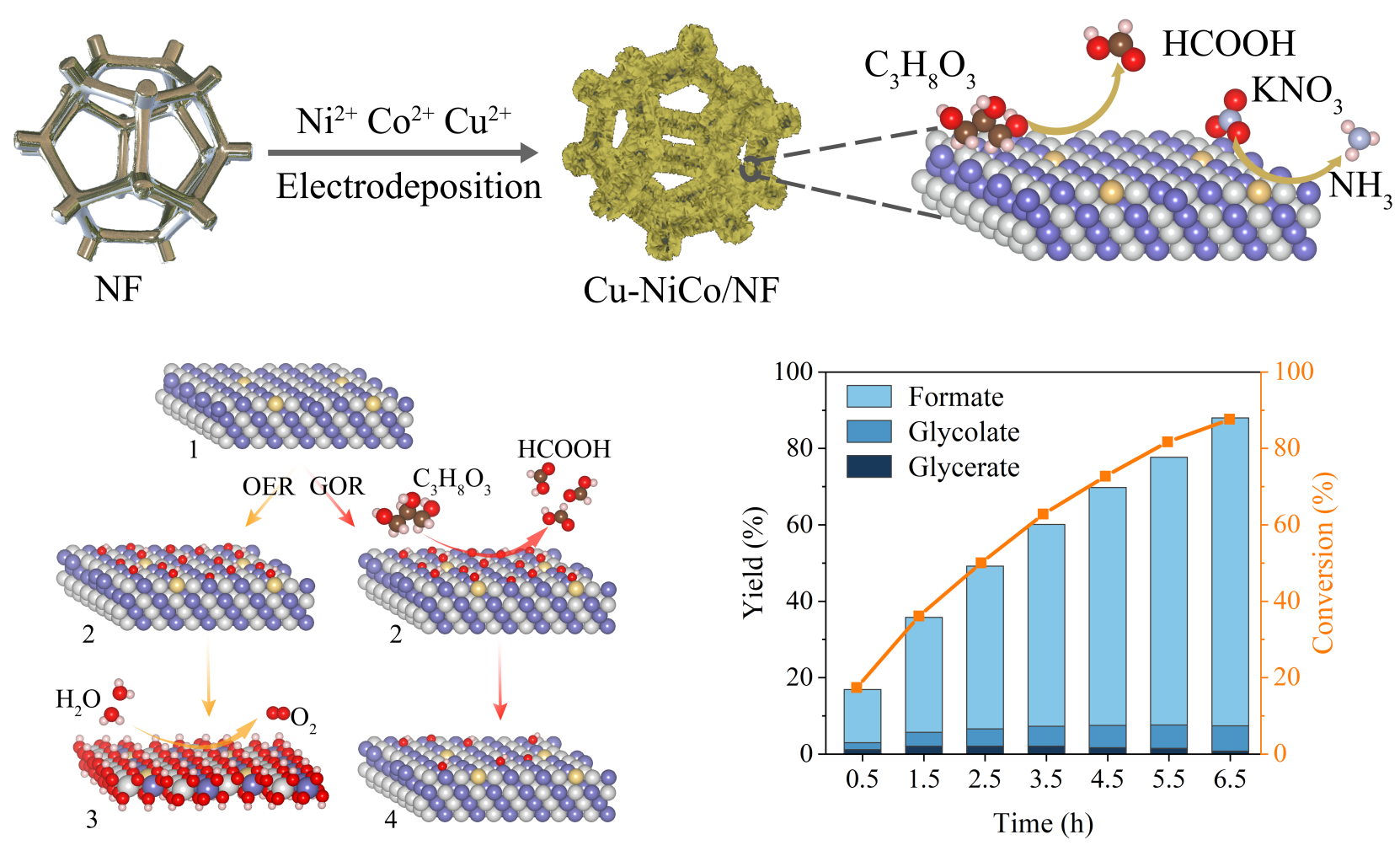
Schematic illustration of the synthesis of Cu-NiCo/NF and the spontaneous GOR on the Cu-NiCo/NF surface, and changes in glycerol conversions and products yields with electrolysis time for the NO3-RR||GOR flow cell (Image by LI Chenyang and LI Hao)
The researchers developed a novel and general high-performance alloy catalyst, Cu-NiCo/NF, for high-yield glycerol electrooxidation. The catalyst, fabricated through one-step electrodeposition, exhibited exceptional glycerol oxidation reaction (GOR) performance, requiring only 1.23 and 1.33 V vs. RHE to achieve 10 and 100 mA cm-2, respectively, while achieving a Faraday efficiency for formate of 93.8%.
The researchers identified that the rapid generation of NiIII-OOH and CoIII-OOH as active species were promptly consumed during GOR, confirming the structural stability of the Cu-NiCo/NF catalyst. Besides, Cu doping in NiCo reduced the energy barrier and △G of the C-O coupling process, resulting in superior performance of electrocatalytic GOR.
Moreover, the researchers demonstrated that the Cu-NiCo/NF catalyst performed high activity and selectivity for the nitrate reduction reaction (NO3-RR). Using Cu-NiCo/NF as a bifunctional catalyst, the researchers established a NO3-RR||GOR system to produce NH3 and formate simultaneously, requiring only 1.11 and 1.37 V to achieve 10 and 100 mA cm-2, respectively. The system exhibited excellent long-term stability for up to 144 hours, with formic acid remaining the primary product at the anode. When the glycerol conversion rate reached 87.6%, the formation yield was 80.6%.
"This work not only develops a bifunctional electrocatalyst for GOR and nitrate reduction with high catalytic activity but also provides a new strategy for electrochemical refinery with product upgrades," said Prof. WU.