Producing valuable multicarbon (C2+) chemicals and fuels like ethylene via catalytic conversion of carbon monoxide (CO) derived from coal, natural gas, and biomass is an important nonpetroleum route. The thermocatalytic CO conversion processes such as Fischer-Tropsch synthesis (FTS) suffer from substantial CO2 emission as well as the formation of undesired methane by-products.
Renewable energy-driven CO electrolysis is an emerging catalytic CO conversion route. This reaction proceeds with water as a hydrogen source under ambient conditions and can electrochemically eliminate the formation of CO2 by applying a negative potential on CO molecules.
Recently, a research team from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved high-rate electrosynthesis of C2+ chemicals and fuels via CO electrolysis.
This study was published in Nature Communications on May 30.
High-rate electrosynthesis of multicarbon chemicals and fuels from carbon monoxide (Image by LI Hefei)
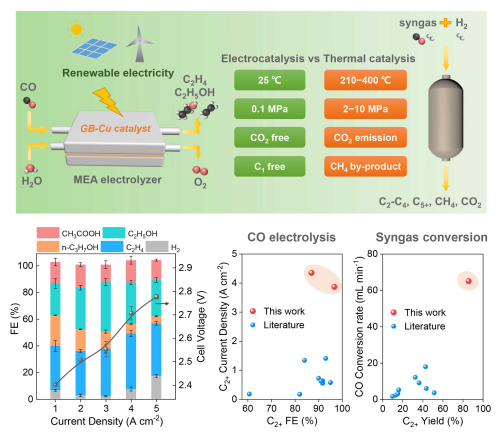
The researchers developed a Cu nanoparticle catalyst with high-density grain boundaries (GBs) via a facile wet-chemistry synthesis approach. In combination with advanced alkaline membrane electrode assembly electrolyzers developed by the team, they achieved a C2+ partial current density as high as 4.35 A cm-2 at a low cell voltage of 2.78 V, with a C2+ Faradaic efficiency of 87% and a notable C2+ yield as high as 85%.
Operando Raman spectroscopy and density functional theory calculation results revealed that the abundant grain boundaries of Cu nanoparticles facilitate CO adsorption and C-C coupling, thus rationalizing the impressive C2+ production.
A scale-up demonstration using the above Cu catalyst and an electrolyzer stack with five 100 cm2 membrane electrode assembly achieved high C2+ and ethylene formation rates of 118.9 mmol min-1 and 1.2 L min-1 at a total current of 400 A, respectively.
"This work demonstrates the great promise of CO electrolysis as a sustainable and practical route for the electrochemical synthesis of valuable C2+ chemicals," said Prof. GAO Dunfeng who is the author of this study. "Our work also highlights the significance of coupling catalyst design and device design in developing highly-efficient electrolysis technology," said Prof. WANG Guoxiong who is another author of this study.
The above work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Liaoning Revitalization Talents Program, and the Youth Innovation Fund of DICP. (Text by GAO Dunfeng)