Terpenoids are widely used in clinical therapeutics and medicinal discovery, as well as play important roles in cosmetic, food and perfume industries. In nature, terpenoids are synthesized from isopentenyl pyrophosphate and dimethylallyl pyrophosphate through multistep enzymes catalysis.
Since terpenoids are important, developing selective synthesis of terpenoids from bulk chemicals by mimicking the biological process is necessary.
Recently, a team led by Prof. CHEN Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a bioinspired and catalytic transformation of isoprene.
Their findings were published in Nature Communications on Nov. 04.
Isoprene is considered as cost-effective choice for biomimetic constructions of terpenoids due to its characteristic structure and abundance.
In terms of the synthesis of hemiterpenoids and acyclic monoterpenoids, various catalytic systems have been well developed under transition metal catalysis. However, there are many challenges, such as chemo-, regio- and redoxselectivities, to realize the nucleophilic cyclictelomerizations of isoprene, especially nucleophilic aromatization.
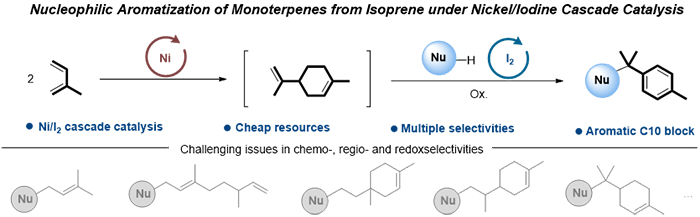
Nucleophilic aromatization of monoterpenes from isoprene (Image by ZHANG Weisong)
In this study, the researchers developed a new strategy to realize nucleophilic aromatization of monoterpenes from isoprene under nickel/iodine cascade catalysis, and it could be applied to various nucleophiles such as indoles, indazoles, benzotriazole and pyrroles.
In this strategy, a series of natural terpenes were transformed to aromatic C10 or C15 blocks by I2 catalysis. Based on the good tolerance of different monoterpenes, the convergent synthesis strategy was implemented smoothly by using drugs and plant extracts.
Meanwhile, the researchers also indicated the possible catalytic pathways of this strategy. Firstly, through the regulation of IPr ligand, the cyclodimerization of isoprene proceeded under Ni catalysis. Then, In the presence of K2S2O8, a highly selective nucleophilic addition occurred quickly following the oxidative aromatization of limonene under I2 catalysis.
Moreover, they realized the orthogonal C–H functionalization of two regioisomers of monoterpene with different nucleophiles in one same pot. And various hybrid terpenyl indoles were programmatically assembled to illustrate the practical utility of this protocol.
The above work was supported by Dalian Outstanding Young Scientific Talent and the National Natural Science Foundation of China.