The in-cell dynamic structures of proteins are crucial for unraveling their biological functions. With the advancement of deep learning algorithms aiding protein structure prediction, AlphaFold2 has achieved comprehensive protein structure prediction.
However, predicting the structures of flexible regions remains unclear.
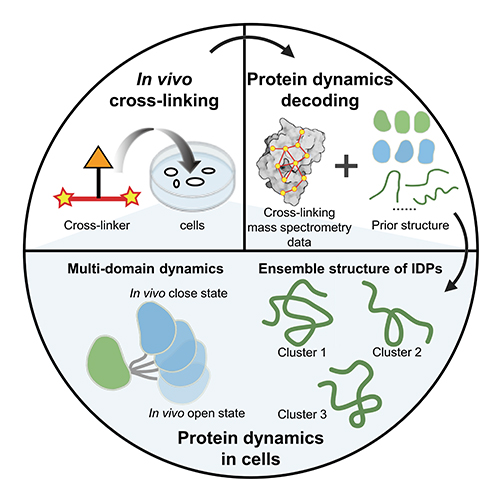
Recently, a group led Prof. ZHANG Lihua from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Associate Prof. GONG Zhou from the Precision Measurement Science and Technology Innovation Research Institute of CAS, have proposed a strategy using in vivo chemical cross-linking and mass spectrometry (in vivo XL-MS) to decode the dynamic structure of proteins in cells.
This study was published in Angewandte Chemie International Edition on July 05.
In vivo XL-MS is potential for analyzing the in-cell dynamic structures of proteins due to its high throughput, high sensitivity, and low requirements for protein purity.
In this study, the researchers utilized AlphaFold2's structures as prior information, combining in vivo XL-MS data with various structural calculation methods to assess the compatibility between structures and cross-linking information. They have achieved the reconstruction of the in-cell dynamic structures of various proteins, especially multi-domain proteins and intrinsically disordered proteins (IDPs).
Schematic illustration of a hierarchical strategy to decode protein dynamics in vivo with XL-MS (Image by ZHANG Beirong)
They focused on multi-domain proteins and proposed a strategy to treat the domains as a whole, utilizing XL-MS data between the domains to model the dynamic structures of proteins inside cells. They characterized the dynamic structures of three multi-domain proteins, namely calmodulin, hnRNP A1, and hnRNP D0, within cells.
Additionally, for IDPs, they introduced two complementary structural characterization strategies: one involved directly converting XL-MS data into distance constraints for IDP structure calculation, while the other strategy employed unbiased sampling using all-atom molecular dynamics simulations, followed by evaluation and selection of the sampled structures based on XL-MS data. By employing these two strategies, they decoded the ensemble conformations of two highly mobile high-mobility group proteins, HMG-I/Y and HMG-17, inside cells.
"Our study provides crucial technical support for a deeper understanding of the molecular mechanisms underlying protein functionality in the cellular microenvironment," said Prof. ZHANG.
This work was supported by the National Natural Science Foundation of China, the National Key R&D Program, the Youth Innovation Promotion Association of CAS.