Catalytic oxygen evolution reaction (OER), providing protons and electrons from water, plays an important role in clean energy storage and conversion processes. Its transformation requires sequential intermediate valence change steps, which makes the dynamics of the catalytical cycle complicated.
Such multi-redox mechanism involving multi-sites has implications to dictate the catalytic water oxidation. However, understanding the sequential dynamics of multi-steps in OER cycles on catalysts is still challenging.
Recently, a research group led by Profs. LI Can and WANG Xiuli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has revealed sequential oxidation kinetics and determining roles of multi-cobalt active sites on Co3O4 catalyst for water oxidation.
The study was published in Journal of the American Chemical Society on February 01.
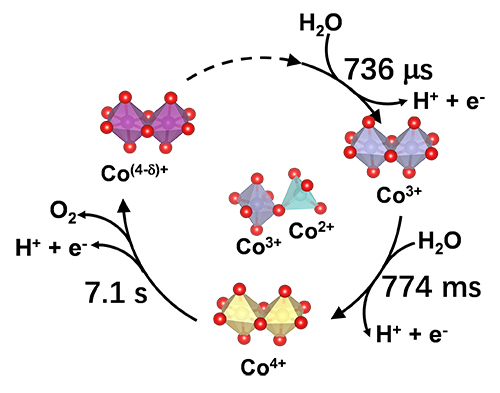
Schematic of the proposed valence change kinetics of OER catalytic cycle on Co3O4 (Image by KANG Wanchao)
The researchers employed quasi-operando transient absorption spectroscopy to study a typical photosensitization strategy, using Co3O4 nanoparticles as model catalysts.
They found that when OER initiated from fast oxidation of surface Co2+ ions, both surface Co2+ and Co3+ ions were active sites of the multi-cobalt centers for water oxidation.
In the sequential kinetics (Co2+ → Co3+ → Co4+), the key characteristic was fast oxidation and slow consumption for all the cobalt species. Due to this characteristic, the Co4+ intermediate distribution played a determining role in OER activity and results in the slow overall OER kinetics.
"Our study deepens the understanding of the multi-redox kinetics of multi-active sites in OER catalytic cycle. It also sheds light on the water oxidation mechanism and heterogeneous OER catalyst design," said Prof. Li.
This work was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the DICP Foundation of Innovative Research.