A research group led by Prof. LI Xianfeng from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a Bromine-assisted-MnO2-based hybrid single flow battery, which exhibits advantages of high energy density and reversibility.
This study was published in Angewandte Chemie International Edition on Oct. 26.
Mn2+/Mn3+ redox pair has been considered as a promising cathode for high-energy-density batteries, due to its attractive features of high redox potential, solubility and outstanding kinetics. However, the disproportionation side reaction of Mn3+, which results in accumulation of "dead" MnO2, limits its reversibility and further energy density.
In this study, the researchers provided a new concept to solve the issue of "dead" MnO2 by introducing Br-/Br2 into Mn2+/MnO2 catholyte in a highly acidic environment.
Br- was firstly oxidized to Br2 during charge, and then Mn2+ was oxidized to Mn3+, which could be partially disproportionated to form MnO2 simultaneously. During discharge, Mn3+ and part of MnO2 were reduced to Mn2+ firstly and Br2 was reduced to Br-. Then the produced Br- could react with "dead" MnO2 to Br2, participating in discharge, completing the reduction process, and avoiding the accumulation of "dead" MnO2.
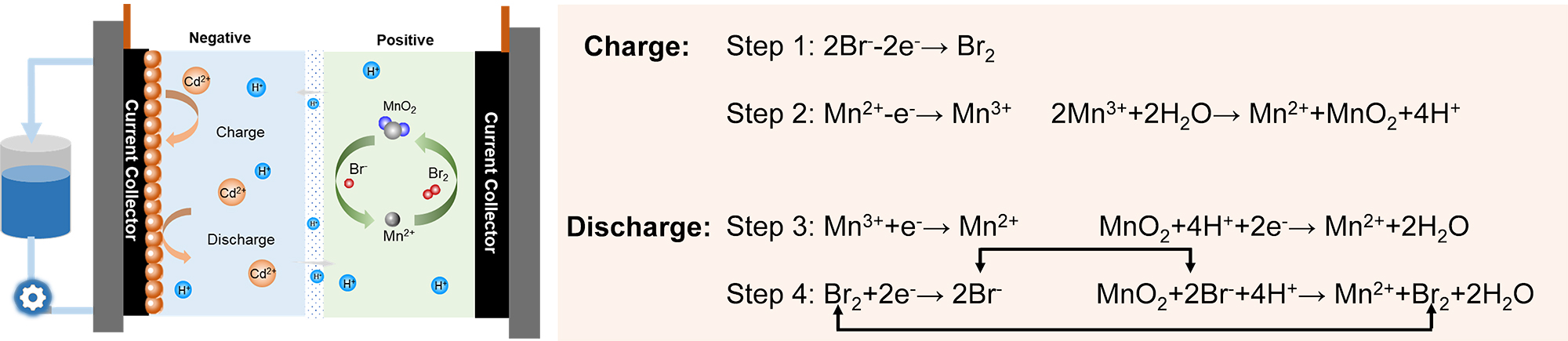
The principle and electrochemical detection of the BMFB, and the summary of the electrochemical mechanism of MnSO4+HBr (Image by XIE Congxin and LIU Yun)
Furthermore, the researchers assembled Bromine-Manganese flow battery (BMFB) coupling with Cd/Cd2+ as anode. The battery exhibited high energy density of 360 Wh L-1 and stably running for over 500 cycles at a current density of 80 mA cm-2.
"The battery assembled with silicotungstic acid as anode could continuously run for over 2000 cycles at 80 mA cm-2, which further confirmed the reliability and universality of the catholyte," said Prof. Li. "We believe that the BMFB has great potential for large-scale energy storage."
This work was supported by the China Natural Science Foundation, the Strategic Priority Research Program of the CAS, the CAS Engineering Laboratory for Electrochemical Energy Storage and CAS-DOE program, and DICP funding. (Text by XIE Congxin and LIU Yun)