A research group led by Prof. GUO Jianping and Prof. CHEN Ping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. WU An'an from Xiamen University, revealed how lithium hydride mediated hydrogenolysis of anilines to arenes.
This study was published in Journal of the American Chemical Society on Sep. 15.
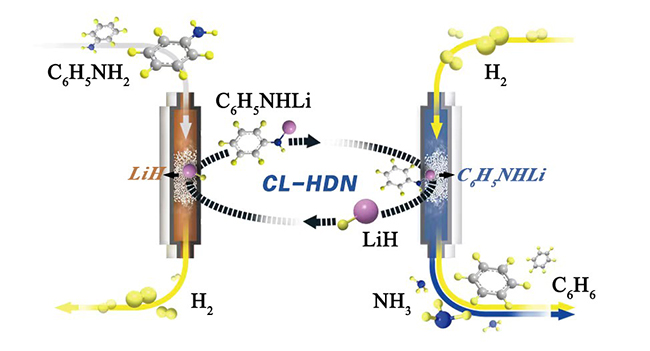
Lithium hydride mediated transition metal-free hydrogenolysis of anilines to arenes (Image by CAI Yongli)
C-N bonds are ubiquitous in organic and biomacromolecules. The activation of C-N bonds is involved in many important chemical processes. Because of the high C-N bond dissociation energy, the intense coordinating ability, and the inferior leaving ability of the NH2 group, the cleavage of C-N bond, specially the sp2C-N bond remains challenging.
The researchers proposed a lithium hydride (LiH)-mediated chemical looping process for the hydrogenolysis of aniline, which decoupled the overall hydrodenitrogenation (HDN) reaction into a set of separated steps.
In the first step of the loop, LiH deprotonated aniline to form a lithium anilide and H2. The lithium anilide was then exposed to dihydrogen at elevated temperatures to produce benzene and lithium amide (LiNH2) in the second step. And in the last step, the LiH was regenerated by the hydrogenation of LiNH2 in a flow of H2, closing the chemical cycle.
They found that benzene was the dominant denitrogenated product in this process, which was in sharp contrast to those of transition metals. This led to the complete hydrogenation of aromatic rings. Therefore, they achieved a highly denitrogenated product formation rate under lower temperatures and pressures, which was comparable to the catalytic rate of transition metal catalysts.
The computational studies revealed that the cleavage of C-N bond was facilitated via a LiH-mediated pathway, in which the hydride (H-) of LiH functioned as a nucleophile to attack the α-sp2C atom and Li cation interacted with the distorted aromatic ring by a cation-π interaction.
"This work provides a new strategy for C-N bond activation and may help the design and development of new materials or catalysts for the HDN as well as other important chemical transformations," said Prof. GUO. (Text by CAI Yongli)