Methane, mainly from natural gas, shale gas and methane hydrate, is one of the most economic fossil fuels. However, it remains a great challenge to realize the selective valorization of methane under mild conditions due to the inherently small polarizability and high dissociation energy of C-H bond in CH4 as well as the higher reactivity of target oxygenates. 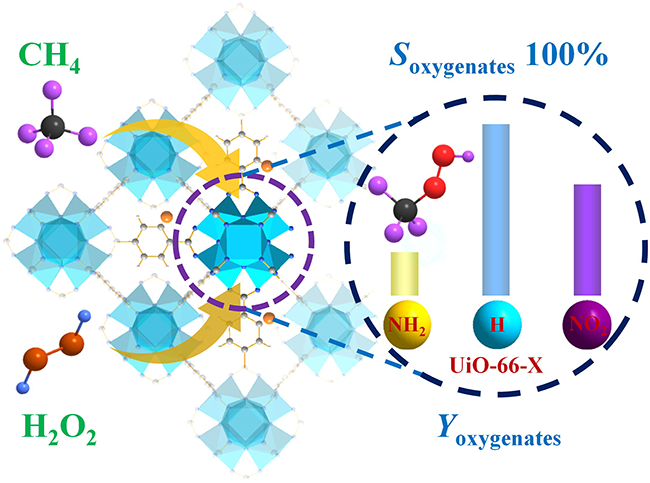
Recently, a research group led by Prof. WANG Xiaodong and Prof. LIN Jian from the Dalian Institute of Chemical Physics(DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. ZHU Chun from Guizhou University, has developed UiO-66 metal-organic frameworks (MOFs) catalysts with tunable electronic property, which could enhance the selective oxidation of methane.
This study was published in Angewandte Chemie International Edition on June 29.

The UiO-66-X catalyst directly convert CH4 to oxygenates with 100% selectivity by using H2O2 as an oxidant (Image by FANG Geqian)
The over-oxidation of target products and the metal sites leaching are usually unavoidable on the supported metal species. Meanwhile, the structured metal-oxo species on bulk metal oxides or frameworks are inferior reactivity, and they just play as a support or co-catalyst to enhance the CH4 conversion.
To solve the above challenges, the researchers developed the sole UiO-66 MOFs catalysts modified by various ligands (NH2-BDC, H2BDC, and NO2-BDC) to directly convert CH4 to oxygenates with 100% selectivity by using H2O2 as an oxidant under mild conditions.
The Zr-oxo nodes with these modifiers exhibited different electronic properties that affected the anchoring of ·OH species to form effective Zroxo-·OH sites, which can promote the activation of the C-H bond of CH4 with the lowest energy barrier over UiO-66-H.
The above work was supported by the National Natural Science Foundation of China, Youth Innovation Promotion Association of CAS, and Dalian Science Foundation for Distinguished Young Scholars. (Text by FANG Geqian)