A research group led by Prof. PIAO Hailong from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. XU Guowang from DICP and Prof. TAN Guang from the First Affiliated Hospital of Dalian Medical University, identified the correlation between deubiquitinase ubiquitin-specific protease 22 (USP22) and abnormal lipidome accumulation in hepatocellular carcinoma (HCC).
They elucidated a USP22-regulated lipogenesis mechanism that involved the peroxisome proliferator-activated receptor gamma (PPARγ) - ATP citrate lyase (ACLY) / acetyl-CoA carboxylase (ACC) axis in HCC tumorigenesis, and provided a rationale for therapeutic targeting of lipogenesis via USP22 inhibition.
This study was published in Nature Communications on April 21.
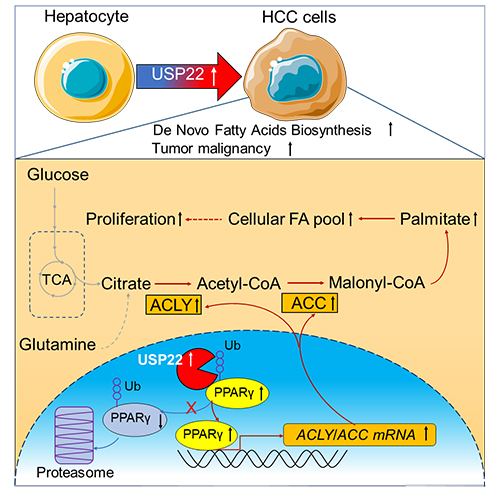
PPARγ is stabilized by USP22-mediated deubiquitination leading to lipid accumulation and promoting hepatocellular carcinoma (Image by NING Zhen)
Metabolic reprogramming is one hallmark of cancer, among which the abnormally increased de novo lipogenesis (DNL) is one of the most common features.
Fatty acid synthesis occurs at a lower rate in nondividing cells, which mainly absorbs lipids from the extracellular circulation. In contrast, DNL, especially de novo fatty acid synthesis, is an important lipid source for cancer cells.
In this study, the researchers detected the expression of USP family members by immunoblotting in HCC tissues compared to paired normal adjacent tissues. They further screened different metabolites using liquid chromatography–mass spectrometry.
The results showed USP22 was the most significantly high expressed USP member, and this high expression of USP22 was correlated with lipids and lipid-like metabolites upregulation.
Molecular and biochemical experiments confirmed USP22 regulated lipid metabolism through PPARγ, a ligand-activated transcription factor belonging to the nuclear hormone receptor family that promoted lipogenesis by upregulating lipid synthesis enzymes such as ACC, ACLY and fatty acid synthase (FASN).
PPARγ was the substrate of USP22 and USP22-mediated deubiquitination stabilized PPARγ and activated PPARγ target genes (i.e., ACC and ACLY), contributing to lipogenesis and HCC tumorigenesis.
"This study provides an option for targeting fatty acid synthesis, which may benefit patients with high USP22 expression in HCC," said Prof. PIAO. (Text by LIU Xiaolong)