Ethylene, as one of the most important building blocks in chemical synthesis, is mainly produced from naphtha cracking at high temperatures of around 800 ℃. Due to China is rich in coal and short of oil, it's importance to develop highly efficient ethylene production route from hydrogenation of coal-based acetylene.
However, traditional thermocatalytic hydrogenation of acetylene to ethylene (HAE) requires high temperatures and high pressure, leading to excessive energy consumption. Besides, large amount of H2 consumption makes this process even more costly.
Recently, a research group led by Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) achieved a highly efficient electrocatalytic hydrogenation of acetylene to ethylene (E-HAE) under room temperature by directly using water as hydrogen source.
The study was published in Nature Communications on December 6.
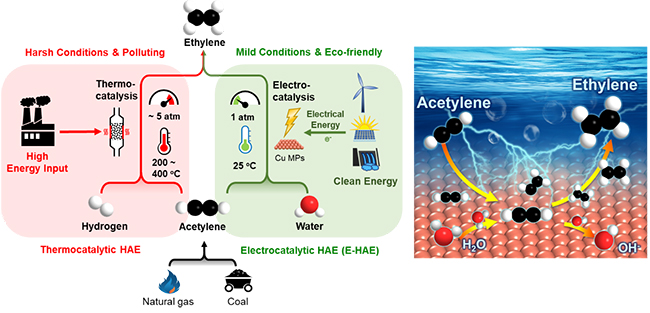
The features of the E-HAE process compared with the traditional thermocatalytic HAE process (Image by WANG Suheng and YU Liang)
In contrast to the thermocatalytic path, the new process developed by the researchers was advantageous in being operatable under mild conditions and was environmental-friendly in combination with renewable energy-based electricity, in which hydrogen was in-situ generated from electroreduction of water so that extra supply of H2 could be avoided.
By optimizing the Cu catalyst to expose more active facets, preferential adsorption and hydrogenation of acetylene was facilitated against hydrogen adsorption and evolution. By using a microporous gas diffusion layer to promote mass transfer, a high Faradaic efficiency of 83.2% for ethylene production was achieved with a current density of 29 mA cm-2 at -0.6 V vs. RHE.
In-situ spectroscopic characterizations combined with density functional theory calculations demonstrated that electron transfer from the Cu surface to adsorbed acetylene promoted the adsorption and hydrogenation of the acetylene, while suppressing the competitive hydrogen evolution reaction and facilitating ethylene desorption, thereby resulting in highly selective ethylene production via the electron-coupled proton transfer pathways.
"This process provides a green route for industrial production of C2H4 from C2H2 under mild conditions," said Prof. DENG.
This work was supported by the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM). (Text by WANG Suheng and YU Liang)