(Dihydro)thiophenes, one of the most common five-membered heterocycles, are widespread in a large number of natural products, functional materials, and biologically active compounds.
Sulfide sources are usually employed to prepare thiophene compounds through the formation of two new C–S bonds. However, the substrates employed are highly functionalized precursors, leading to limited scope and functional group compatibility.
Recently, a research team led by Prof. CHEN Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed the redoxdivergent construction of (dihydro)thiophenes with dimethyl sulfoxide (DMSO) as both an oxidant and a sulfur donor.
This study was published in Angewandte Chemie International Edition on August 30.
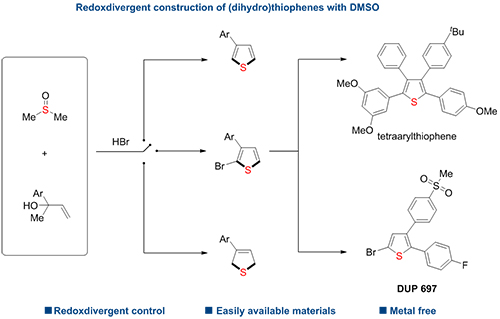
Redoxdivergent construction of dihydrothiophenes, thiophenes and bromothiophenes respectively from readily available allylic alcohols,DMSO and HBr (Image by LIU Heng)
The researchers employed readily available allylic alcohols as starting materials and DMSO as mild oxidant to offer derivatives of (dihydro)thiophenes efficiently. They found that the manipulation of the selectivity could be governed by the dosage of DMSO and HBr.
In addition, they demonstrated that this strategy could realize programmable and concise synthesis of tetraarylthiophenes, bioactive DuP 697 and its regioisomers. It may serve as a general platform for achieving synthetically and medicinally useful five-numbered sulfur-containing heterocycles.
The research was supported by Dalian Institute of Chemical Physics, Dalian Outstanding Young Scientific Talent and the National Natural Science Foundation of China. (Text by Heng Liu)