Nicotinamide adenine dinucleotide (NAD), an indispensable cofactor in cells, participates in diverse redox biochemistry and other nonredox processes. NAD level variation leads to global yet difficult-to-predict biological responses.
A research team led by Prof. ZHAO Zongbao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences has committed to developing non-natural cofactor-based systems to avoid the limitation associated with NAD level fluctuation.
The team has designed a non-natural coenzyme nicotinamide cytosine dinucleotide (NCD), and engineered a series of oxidoreductases. However, it's still challenging for microbial cells to take NCD from the environment.
Recently, the researchers created the synthetase for NCD and established NCD self-sufficient microbial phenotype for metabolic regulation.
This study was published on Nature Communication on April 9.
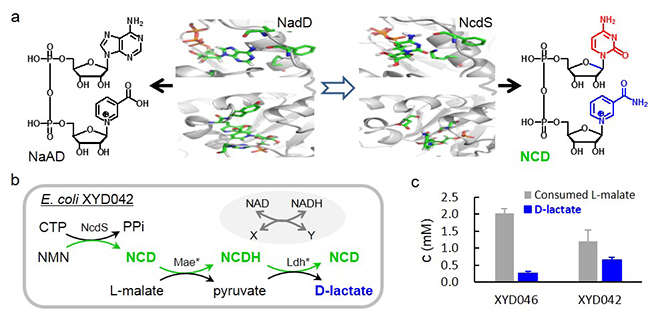
Created NcdS for NCD biosynthesis and the results of NCD-linked D-lactate accumulation (Image by WANG Xueying)
The researchers reprogrammed the ATP and nicotinic acid mononucleotide (NaMN) binding pockets of NaMN adenylyltransferase (NadD), the key enzyme in NAD biosynthesis, to favor CTP and nicotinamide mononucleotide (NMN).
"We created novel NCD synthetases (NcdSs) by combinatorial mutation of both key residues," said Prof. ZHAO.
Furthermore, they constructed NCD self-sufficient strains with different gradient NCD levels by precursor enhancement and pathway optimization. And the NCD level was up to 5.0 mM, higher than natural level of NAD.
They found that NCD synthesized in-situ could drive the selective conversion of L-malic acid to D-lactate in the presence of NCD-dependent malic enzyme mutant and D-lactate dehydrogenase mutant. NcdS together with NCD-linked enzymes may offer unique tools for intriguing studies in chemical biology and synthetic biology.
This work was supported by National Key R&D Program of China, National Natural Science Foundation of China. (Text by WANG Xueying)