Lysine residue (K) bears a positively charged amino group on its side chain under the native physiological statuses of proteins. It can interact with a negatively charged amino acid or anionic ligands to form salt bridges or hydrogen bonds, which is essential for stabilizing the protein structure and keeping the protein activity.
Recently, a team led by Prof. WANG Fangjun from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a mass spectrometry (MS)-based two-step isotope labeling-lysine reactivity profiling (TILLRP) strategy to quantify the reactivity of lysine residues and probe the molecular details of protein-protein interactions as well as evaluate the conformational interventions by small-molecule active compounds.
This study was published in Chemical Science on Nov. 23.
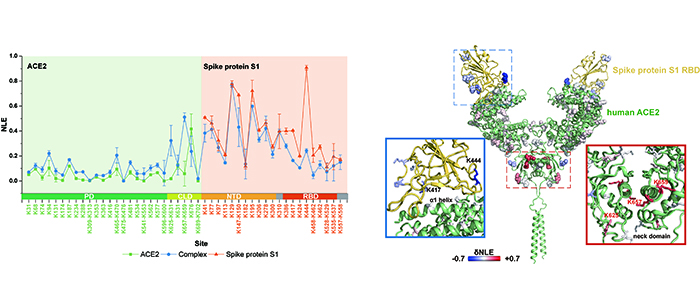
Quantitative lysine reactivity profiling of S1-ACE2 before and after the formation of complex (Image by LIU Zheyi)
Many lysine residues are protein function centers and involved in protein structure modulation and biological function regulation.
Previously, Prof. WANG’s lab developed a series of new methods for lysine reactivity profiling and applied these methods in monitoring the protein-protein, receptor-ligand, and kinase-inhibitor interaction details, including both the direct interaction regions and conformation modulated regions.
In this study, they applied the TILLRP strategy to monitor the dynamic conformational hotspots of the recognition and intervention of SARS-CoV-2 S1 with ACE2 receptor.
"We discovered the labeling reactivity of lysine residues in the complex interaction interface of S1-RBD Lys386-Lys462 were directly related with the protein complex formation, and it might be utilized as indicators for investigating the S1-ACE2 dynamic recognition and intervention," said Prof. WANG.
This TILLRP strategy exhibits the capability in probing and evaluating the dynamic conformational alterations of S1-ACE2 recognition and intervention by exogenous compounds at molecular level in high throughput. (Text by LIU Zheyi & ZHANG Wenxiang)