Strong metal-support interaction (SMSI) effect is one of the most important concepts in heterogeneous catalysis. This effect is almost exclusively observed in metal catalysts supported on active and reducible oxides, in which the support-derived material can encapsulate metal nanoparticles under certain treatment or reaction conditions.
A research team led by Prof. FU Qiang and Prof. BAO Xinhe from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences (CAS) reported the SMSI effect in transition metal catalysts supported on hexagonal boron nitride (h-BN) sheets.
The results were published in J. Am. Chem. Soc. on September 14.
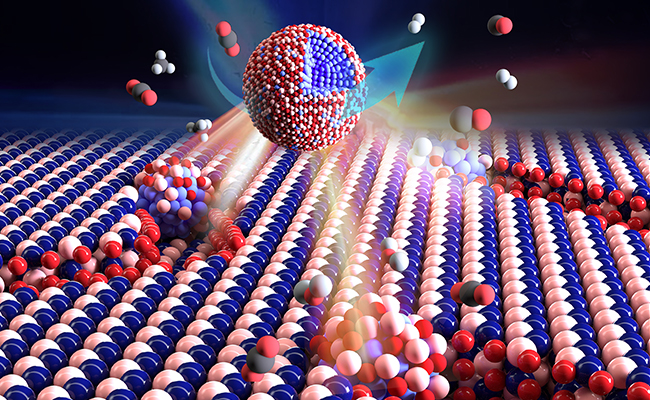
Formation of etching nanopits in h-BN sheets aided by Ni particles and encapsulation of the Ni particles by BOx overlayers during dry reforming of methane reaction.(Image by DONG Jinhu)
The scientists observed classical SMSI process between metal nanoparticles, e.g., Ni, Fe, Co, and Ru, and inert hexagonal boron nitride (h-BN) nanosheets.
Weak oxidizing gases such as CO2 and H2O induced the encapsulation of Ni nanoparticles by ultrathin boron oxide (BOx) overlayers derived from the h-BN support during the dry reforming of methane (DRM) reaction.
Experimental and theory calculation results showed that the etched nanopits at the Ni/h-BN interface could confine the Ni nanoparticles against sintering. The amorphous BOx encapsulation overlayers were permeable for the reactants and work synergistically with Ni. Both factors promote the DRM reaction.
This work was supported by the National Natural Science Foundation of China, the Strategic Priority Research Program (B) of the Chinese Academy of Sciences, and the Ministry of Science and Technology of China. (Text by DONG Jinhu)